(E)-1-acetoxy-2,7-octadiene | 30460-73-2
中文名称
——
中文别名
——
英文名称
(E)-1-acetoxy-2,7-octadiene
英文别名
1-acetoxy-2,7-octadiene;2,7-octadienyl acetate;trans-2,7-octadienyl acetate;1-Acetoxyoctadiene;[(2E)-octa-2,7-dienyl] acetate
CAS
30460-73-2
化学式
C10H16O2
mdl
——
分子量
168.236
InChiKey
RDOHPVPONNHSPH-BQYQJAHWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:225.0±19.0 °C(Predicted)
-
密度:0.905±0.06 g/cm3(Predicted)
-
保留指数:1388
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:12
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:(E)-1-acetoxy-2,7-octadiene 在 tris(dibenzylideneacetone)dipalladium(0) chloroform complex 、 三丁基膦 甲酸铵 作用下, 以 1,4-二氧六环 为溶剂, 反应 2.0h, 以100%的产率得到1,7-辛二烯参考文献:名称:钯催化末端烯丙基化合物与甲酸铵的区域选择性合成1-烯烃摘要:以钯-三丁基膦配合物为催化剂,烯丙基酯、苯基醚、碳酸酯、氯化物和乙烯基环氧化物等各种末端烯丙基化合物与甲酸铵或甲酸钠反应生成具有高区域选择性的1-烯烃。该反应提供了一种有用的 1-烯烃合成方法。DOI:10.1246/cl.1984.1017
-
作为产物:描述:(2E)-octa-2,7-dien-1-ol 、 乙酸酐 在 4-二甲氨基吡啶 、 三乙胺 作用下, 以 二氯甲烷 为溶剂, 以97%的产率得到(E)-1-acetoxy-2,7-octadiene参考文献:名称:Diastereocontrol in Asymmetric Allyl–Allyl Cross-Coupling: Stereocontrolled Reaction of Prochiral Allylboronates with Prochiral Allyl Chlorides摘要:Palladium-catalyzed allyl-ally cross-coupling was investigated with substituted prochiral allylic boronates. These reactions deliver products bearing adjacent stereocenters, and the issue of diastereocontrol is therefore paramount. Under appropriately modified conditions, this allyl allyl coupling strategy was found to apply to a range of substrates, generally occurring with high enantioselectivity (92:8 to >99:1 er) and good diastereoselection (4:1 to 14:1 dr).DOI:10.1021/ja2075967
文献信息
-
Oxidation of olefins to ketones in combination with electrooxidation作者:Jiro Tsuji、Makoto MinatoDOI:10.1016/s0040-4039(00)96354-8日期:1987.1In combination with anodic oxidation, 1-alkenes are oxidized to methyl ketones efficiently by the catalyses of Pd(OAc)2 and benzoquinone. Cyclopentene and Cyclohexene are oxidized smoothly to the corresponding ketones in high yields.
-
Synthesis of (±)-dimethyl curvularin based on the palladium-catalyzed carbonylation of 3,5-dimethoxybenzyl chloride using a butadiene telomer as a building block作者:Takashi Takahashi、Hiroshi Ikeda、Jiro TsujiDOI:10.1016/0040-4039(80)80207-3日期:1980.1afforded benzyl 7-(3,5-dimethoxyphenylacetoxy)octanoate (6) in 70% yield, which is the precursor of Curvularin (4). The ester (12) was easily prepared from the butadiene telomer obtained by the palladium-catalyzed reaction of butadiene with acetic acid.
-
Preparation of 1-Alkenes by the Palladium-Catalyzed Hydrogenolysis of Terminal Allylic Carbonates and Acetates with Formic Acid-Triethylamine作者:Jiro Tsuji、Ichiro Minami、Isao ShimizuDOI:10.1055/s-1986-31723日期:——A useful method for the preparations of 1-alkenes from terminal allylic carbonates and acetates by the palladium-catalyzed reaction with formates is described. Formic acid-triethylamine is a suitable reductant. As catalyst, Pd2(dba)3CHCl3-P(n-Bu)3, gave the best results. 0.05 0.2mol% being sufficient (turnover 500 2000). Using this method, various 1-alkenes were prepared in good yields with high selectivity.
-
Total synthesis of lipoxin A4 and lipoxin B4 from butadiene作者:A Rodrı́guez、M Nomen、B.W Spur、J.J Godfroid、T.H LeeDOI:10.1016/s0040-4039(99)02201-7日期:2000.2The total synthesis of LXA4 and LXB4 has been achieved starting from butadiene via palladium catalyzed telomerization. Sharpless catalytic AE and C-2 inversion of the 2(S),3(S)-epoxy alcohols, using Myers CO2/Cs2CO3 procedure, generated the asymmetric centers. The flexibility of the strategy allows an easy access to the linear eicosanoids.
-
Method of producing highly unsaturated compounds by reacting申请人:Mitsubishi Chemical Industries Ltd.公开号:US03981907A1公开(公告)日:1976-09-21A 1,3-conjugated diene compound and a derivative of a butadiene dimer are subjected to an addition reaction over a rhodium compound. The 1,3-conjugated diene compound adds to the derivative in a molar ratio of 1:1. The derivative may be an ester, ether or alcohol. Said derivative is obtained either by reacting a carboxylic acid, an alcohol or a phenol with a butadiene over a palladium catalyst or by hydrolyzing the reaction product.
表征谱图
-
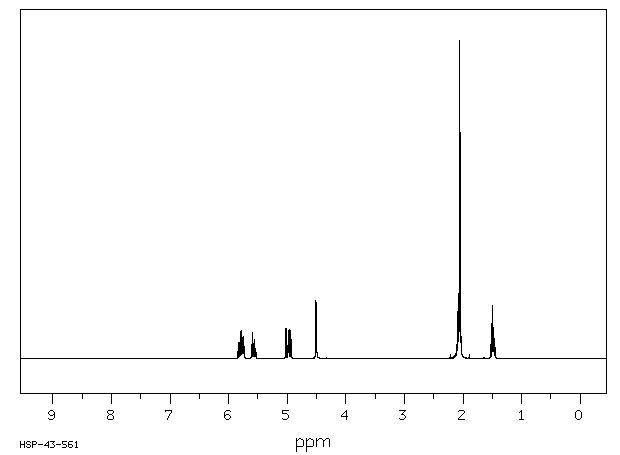
氢谱1HNMR
-
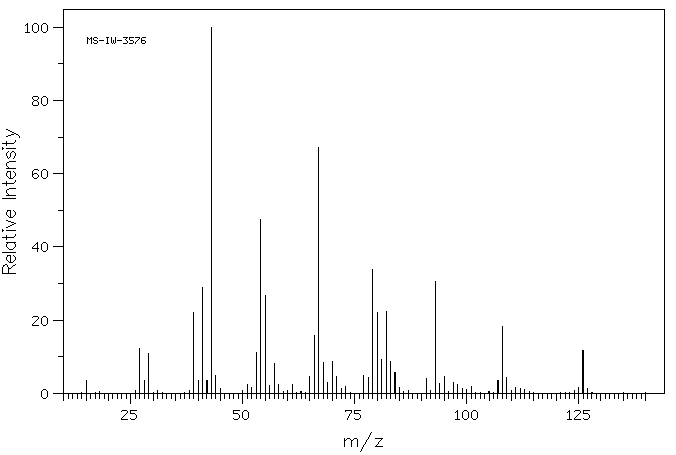
质谱MS
-
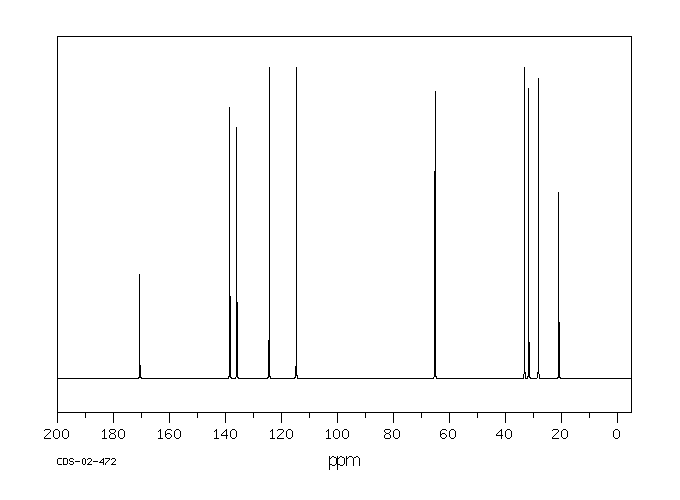
碳谱13CNMR
-
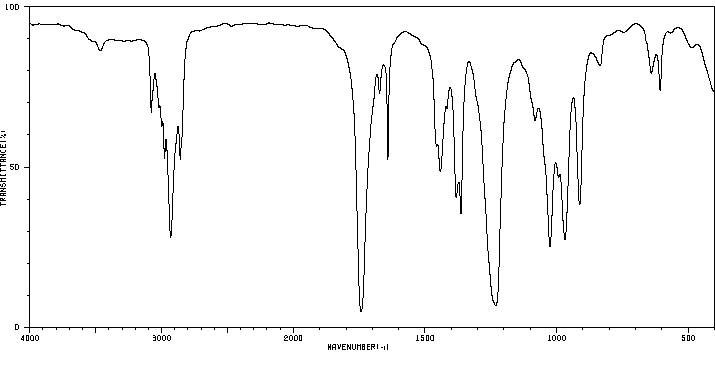
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯