2-(p-Anisidino)tropone | 118818-41-0
中文名称
——
中文别名
——
英文名称
2-(p-Anisidino)tropone
英文别名
2-(4-methoxyanilino)cyclohepta-2,4,6-trien-1-one
CAS
118818-41-0;1265478-34-9
化学式
C14H13NO2
mdl
——
分子量
227.263
InChiKey
BSTMKBHGUSVLCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:3
反应信息
-
作为反应物:描述:2-(p-Anisidino)tropone 、 苄胺 在 triethyloxonium fluoroborate 、 三乙胺 作用下, 以 二氯甲烷 为溶剂, 反应 12.5h, 生成 8-Benzyl-9,9-difluoro-10-(4-methoxyphenyl)-10-aza-8-azonia-9-boranuidabicyclo[5.3.0]deca-1,3,5,7-tetraene参考文献:名称:新型荧光团:硼氨基troponimines的合成和光物理研究摘要:描述了新型荧光硼-氨基troponimine配合物的合成和光物理研究。硼配合物之一的化学结构已通过单晶X射线分析得到了证实,该分析表明在硼处出现了扭曲的四面体几何形状。这些配合物的光物理研究表明,硼-氨基troponimines是荧光分子,其量子产率约为1。0.17–0.28。DOI:10.1016/j.dyepig.2016.10.051
-
作为产物:描述:参考文献:名称:2-Arylhydrazinotropones 的合成和反应。I. 2-(2-Arylhydrazino)tropones 和 4-取代衍生物的制备摘要:通过 2-甲苯磺酰氧托酮与芳基肼反应制备了多种 2-(2-芳基肼基) 托酮。二聚化合物 6,6'-双(芳基肼基)-1,1'-双(2,4-环庚二烯)-7,7'-二酮、2-苯胺托酮和 3-(2-芳基肼基)托酮被分离为在某些情况下是次要产品。类似地,以 2-tosyloxytropones 开始,在 5-位带有异丙基、异丙烯基、(1-乙酰氨基乙基)和受保护的乙酰基,通过异常取代获得 4-取代的 2-(2-芳基肼基)tropones 作为主要产物反应。预计这些 2-(2-芳基肼基) 托酚酮可有效地用作通过联苯胺型重排方便合成 5-芳基取代的托酚酮的前体。DOI:10.1246/bcsj.61.2531
文献信息
-
Prototropic tautomerism and solid-state photochromism of N-phenyl-2-aminotropones作者:Yasuhiro Ito、Kiichi Amimoto、Toshio KawatoDOI:10.1016/j.dyepig.2010.06.001日期:2011.6N-(4-X-phenyl)-2-aminotropones (X = methoxy, chloro, or nitro substituent) and their clathrate crystals with deoxycholic acid (DCA) were prepared and their prototropic phenomena were determined. Each 2-aminotropone derivative existed as a keto amine form in the crystal state irrespective of its substituent, which influenced the stability of keto amine or enol imine structure in solution. X-Ray crystal structure analysis of N-(4-methoxyphenyl)-2-aminotropone revealed the existence of bimolecular hydrogen bonds and close molecular packing, which would inhibit structural change necessary to photo-induced prototropic tautomerization. Thus, N-phenyl-2-aminotropones did not show photocoloration in the crystal state, while their clathrate crystals with DCA were found to exhibit photochromism. (C) 2010 Elsevier Ltd. All rights reserved.
-
KIKUCHI K.; MAKI G.; SATO K., BULL. CHEM. SOC. JAP., 1978, 51, NO 8, 2338-2341作者:KIKUCHI K.、 MAKI G.、 SATO K.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
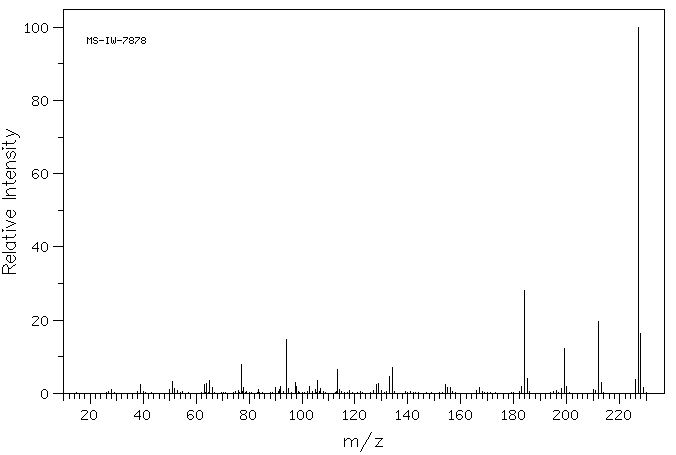
质谱MS
-
碳谱13CNMR
-
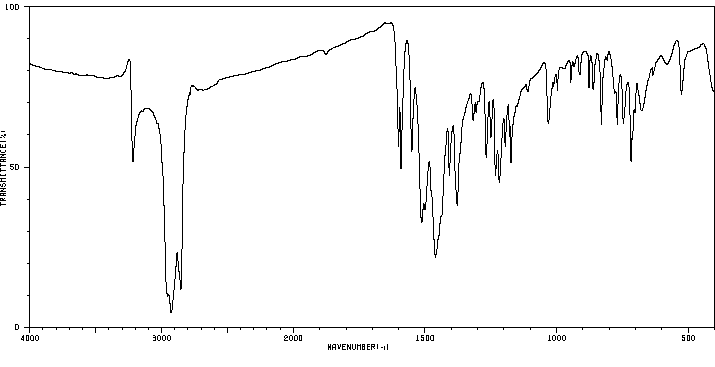
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯