5-(甲氧基)2-(5H)-二氧三环酮 | 112837-17-9
中文名称
5-(甲氧基)2-(5H)-二氧三环酮
中文别名
(R)-(+)-5-(羟甲氧基)-2(5H)呋喃酮
英文名称
(R)-(+)-5-(hydroxymethyl)furan-2(5H)-one
英文别名
(+)-(R)-5-hydroxymethyl-2(5H)-furanone;(R)-(+)-5-hydroxymethyl-5H-furan-2-one;(5R)-5-hydroxymethyl-2(5H)-furanone;(R)-5-hydroxymethyl-5H-furan-2-one;(R)-(+)-5-Hydroxymethyl-2 (5H)-furanone;(R)-(+)-5-(Hydroxymethyl)-2(5H)-furanone;(2R)-2-(hydroxymethyl)-2H-furan-5-one
CAS
112837-17-9
化学式
C5H6O3
mdl
——
分子量
114.101
InChiKey
AWNLUIGMHSSXHB-SCSAIBSYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:39-45 °C
-
沸点:334.8±17.0 °C(Predicted)
-
密度:1.294±0.06 g/cm3(Predicted)
-
稳定性/保质期:
遵照规定使用和储存则不会分解。
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
WGK Germany:3
-
海关编码:2932209090
-
安全说明:S22,S24/25
-
储存条件:存放于阴凉干燥处。
SDS
| Name: | (R)-(+)-5-(Hydroxymethyl)-2(5H)-furanone 98% Material Safety Data Sheet |
| Synonym: | 2(5H)-Furanone, 5-(hydroxymethyl)-, (5R)-; 2(5H)-Furanone, 5-(hydroxymethyl)-, (R)- |
| CAS: | 112837-17-9 |
Synonym:2(5H)-Furanone, 5-(hydroxymethyl)-, (5R)-; 2(5H)-Furanone, 5-(hydroxymethyl)-, (R)-
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 112837-17-9 | (R)-(+)-5-(Hydroxymethyl)-2(5H)furanon | 98 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. This material in sufficient quantity and reduced particle size is capable of creating a dust explosion.
Extinguishing Media:
Use extinguishing media most appropriate for the surrounding fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 112837-17-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 39 - 45 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C5H6O3
Molecular Weight: 114.10
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 112837-17-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
(R)-(+)-5-(Hydroxymethyl)-2(5H)furanone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 112837-17-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 112837-17-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 112837-17-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (+)-(R)-umbelactone 69534-86-7 C6H8O3 128.128
反应信息
-
作为反应物:参考文献:名称:系链控制的exo-selective跨环Diels-Alder(TADA)反应。摘要:[反应:参见正文]从非外消旋的丁烯内酯6开始,描述了一种完全由底物控制的立体选择性途径来构建顺式六氢萘4。关键步骤是四烯5的外选择性跨环Diels-Alder反应(TADA),其内在约束使得选择性地形成一种立体定义的产物。化合物4是合成新型抗生素布雷尼霉素(1)的关键中间体。DOI:10.1021/ol061510m
-
作为产物:描述:L-古洛糖酸-gamma-内酯 在 盐酸 、 sodium periodate 、 硫酸 、 sodium hydroxide 作用下, 以 乙醇 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 21.5h, 生成 5-(甲氧基)2-(5H)-二氧三环酮参考文献:名称:Branimycin的全合成:进化方法摘要:已经描述了大内酯抗生素布雷尼霉素的首次全合成(4)。关键的分离导致顺式-脱氢十氢萘酮核和聚酮化物侧链,它们通过有机金属加成连接。十氢癸酮核心通过总共五种不同的方法作为目标,这些方法具有各种手性元素和闭环方法。最后,选择了最成功的方法,从二环氧萘109开始进行合成。因此,通过有机金属脱对称反应引入氧官能团和碳附件,以生成环氧乙烷107,乙烯基碘化物11在环氧乙烷107上生成。转化成有机锂物质后加入有机硅。通过经由迈克尔加成和随后的大内酯化引入酯侧链来完成合成。DOI:10.1002/chem.201200257
文献信息
-
Tetrahydronaphthofuranone derivatives and process for producing the same申请人:Meiji Seika Kaisha, Ltd.公开号:US06255339B1公开(公告)日:2001-07-03Compounds represented by the following formula (I) and pharmaceutically acceptable salts thereof are disclosed. The compounds have progesterone receptor binding inhibitory activity and, hence, can be used as therapeutic and prophylactic agents for progesterone-related diseases. Specifically, they are useful as abortifacients, oral contraceptive pills, carcinostatic agents for breast cancer and ovarian cancer, therapeutic agents for endometriosis, meningioma, and myeloma, and therapeutic and prophylactic agents for osteoporosis and climacteric disturbance. wherein R1 and R2 represent a hydroxyl group, alkyloxy, alkenyloxy, alkynyloxy, cycloalkyloxy, alkoxyalkyloxy, cycloalkyloxy containig one oxygen atom, aralkyloxy, alkylcarbonyloxy, alkenylcarbonyloxy, alkynylcarbonyloxy, cycloalkylcarbonyloxy, alkoxycarbonyloxy, aryloxy carbonyloxy, aralkylcarbonyloxy, aromatic acyloxy, heteroaromatic acyloxy, saturated heterocyclic carbonyloxy, alkylsulfonyloxy, aromatic sulfonyloxy, alkylcarbamoyloxy, aromatic carbamoyloxy, alkylcarbonylamino, or aromatic acylamino, provided that R1 may further represent a hydrogen atom, alkyl, alkenyl, or alkynyl; and R3, R4, and R5 each independently represent a hydrogen atom, alkyl or alkenyl.以下式(I)表示的化合物及其可药用的盐被披露。这些化合物具有抑制孕酮受体结合的活性,因此可以用作治疗和预防与孕酮相关疾病的药物。特别是,它们可用作堕胎药、口服避孕药、用于乳腺癌和卵巢癌的抗癌剂、用于治疗子宫内膜异位症、脑膜瘤和多发性骨髓瘤的治疗剂,以及用于治疗骨质疏松症和更年期障碍的治疗和预防药物。 其中 R1和R2代表羟基、烷氧基、烯氧基、炔氧基、环烷氧基、烷氧基烷氧基、含有一个氧原子的环烷氧基、芳烷氧基、烷基碳酰氧基、烯基碳酰氧基、炔基碳酰氧基、环烷基碳酰氧基、烷氧基碳酰氧基、芳氧基碳酰氧基、芳烷基碳酰氧基、芳香酰氧基、杂芳酰氧基、饱和杂环碳酰氧基、烷基亚磺酰氧基、芳香亚磺酰氧基、烷基氨基甲酰氧基、芳香氨基甲酰氧基、烷基碳酰胺基或芳香酰氨基,条件是R1还可以进一步代表氢原子、烷基、烯基或炔基;R3、R4和R5各自独立地代表氢原子、烷基或烯基。
-
Synthetic Study on Gymnodimine: Highly Stereoselective Construction of Substituted Tetrahydrofuran and Cyclohexene Moieties作者:Jun Ishihara、Jun Miyakawa、Takashi Tsujimoto、Akio MuraiDOI:10.1055/s-1997-1075日期:——The synthetic studies on gymnodimine, a shellfish toxin, are described. This marine toxin consists of 16-membered carbocycle, tetrahydrofuran, and spiro-imine moieties. Our synthetic strategy involves the stereoselective allylation of tetrahydrofuran compound and the exo-selective intramolecular Diels-Alder reaction.
-
Stereoselective Triplet-Sensitised Radical Reactions of Furanone Derivatives作者:Rabih Jahjah、Abdoulaye Gassama、Véronique Bulach、Chikako Suzuki、Manabu Abe、Norbert Hoffmann、Agathe Martinez、Jean-Marc NuzillardDOI:10.1002/chem.200903045日期:2010.3.15intramolecular reaction, overall, a pyranyl group adds to the α position of the furanone. The effect of conformation was first investigated with compounds 9 a,b carrying an additional substituent on the tether between the furanone and pyranyl moiety. Further information on the effect of conformation and the relative configuration at the pyranyl anomeric centre and the furanone moiety was obtained from已经研究了呋喃酮衍生物的三重态敏化自由基反应的立体选择性和区域选择性。呋喃酮7,b被激发到3通过从丙酮三线态能量传递ππ*状态。然后发生分子内氢提取,使得氢从四氢吡喃转移至呋喃酮部分的β位置。四氢吡喃基和氧代烯丙基的自由基结合产生最终产物8a,b。总体而言,在分子内反应中,吡喃基加至呋喃酮的α位。首先用化合物9a,b研究了构象的影响在呋喃酮和吡喃基部分之间的系链上带有附加的取代基。从葡萄糖衍生物的转化得到上的构象的影响,并在异头吡喃中心的相对构型和呋喃酮基部分进一步信息12,14,17和18。自由基的提取发生在异头异构体中心和葡萄糖基部分的5'-位置。吸氢步骤的计算研究是通过模型结构进行的。计算了此步骤对不同立体异构体的激活势垒,并计算了在四氢吡喃基部分的端基异构中心和6'-位置的抽象。该调查的结果与实验观察结果一致。此外,他们揭示了反应性和区域选择性主要是在吸氢步骤中确定的。分子内夺氢在
-
A Pyrrolysine Analogue for Protein Click Chemistry作者:Tomasz Fekner、Xin Li、Marianne M. Lee、Michael K. ChanDOI:10.1002/anie.200805420日期:——Ignoring the STOP sign: A pyrrolysine analogue bearing a terminal alkyne was site‐specifically incorporated into recombinant calmodulin (CaM) through a UAG codon. The resulting protein was labeled with an azide‐containing dye using a copper(I)‐catalyzed click reaction. Subsequent application of an orthogonal cysteine tagging method yielded a CaM labeled with two distinct fluorophores that enabled its
-
Stereoselective Rhodium-Catalyzed Conjugate Addition of Boronic Acids to Unprotected δ-Hydroxy-γ-butenolides. Synthesis of (−)-7-Oxamuricatacin and β-Substituted Derivatives作者:Cristina Navarro、Ana Moreno、Aurelio G. CsákÿDOI:10.1021/jo8022395日期:2009.1.2The chiral δ-hydroxy-γ-butanolide moiety is widely found among biologically active natural products. We report herein the stereoselective synthesis of β-substituted analogues of these compounds by the RhI-catalyzed conjugate addition of boronic acids to chiral δ-hydroxy-γ-butenolides, easily prepared from the chiral pool. The reaction takes place with high trans diastereoselectivity without protection
表征谱图
-
氢谱1HNMR
-
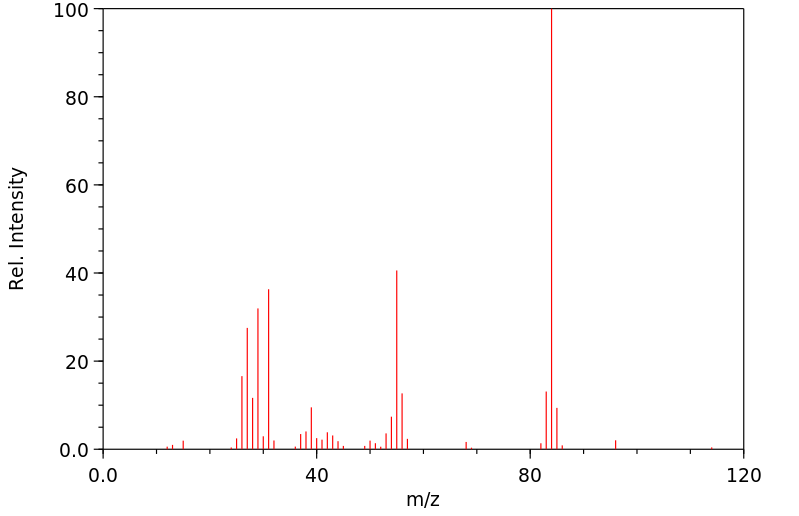
质谱MS
-
碳谱13CNMR
-
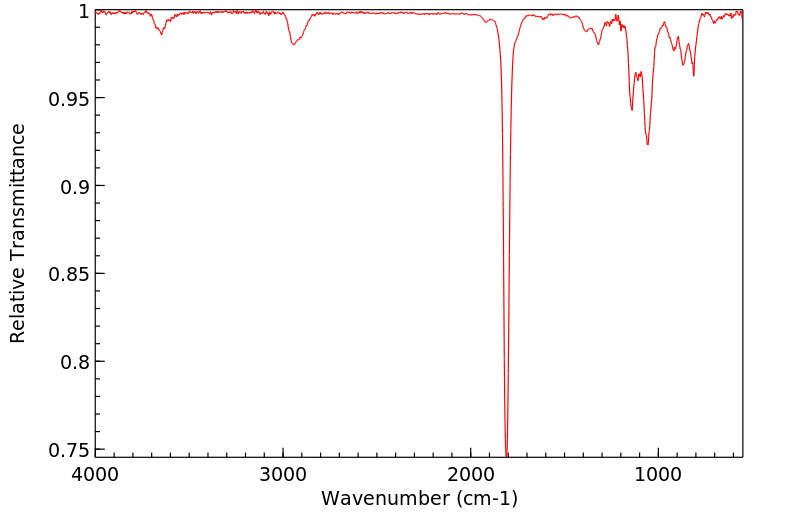
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2R)-4-十六烷酰基-3-羟基-2-(羟甲基)-2H-呋喃-5-酮
马来酸酐-丙烯酸共聚物钠盐
马来酸酐-d2
马来酸酐-13C4
马来酸酐-1-13C
马来酸酐
顺丁烯酸酐-2,3-13C2
顺丁烯二酐与2,2-二甲基-1,3-丙二醇和1,2-丙二醇的聚合物
雄甾-3,5,9(11)-三烯-17-酮,3-甲氧基-(8CI,9CI)
阿西弗兰
阻垢分散剂
钾抗坏血酸盐
重氮基烯,二环[2.2.1]庚-1-基(1,1-二甲基乙基)-,(Z)-(9CI)
赤藻糖酸钠
螺甲螨酯代谢物 M01
葫芦巴内酯
葡糖型抗坏血酸
苯基顺酐
聚氧乙烯(2-甲基-2-丙烯基)甲基二醚-马来酸酐共聚物
聚乙烯马来酸酐共聚物
聚(甲基乙烯基醚-ALT-马来酸酐)
聚(异丁烯-马来酸酐)
聚(乙烯-co-丙烯酸乙酯-co-顺丁烯二酐)
聚(乙烯-co-丙烯酸丁酯-co-马来酸酐)
维生素C钠
维生素C磷酸酯钠
维生素C磷酸酯
维生素C杂质
维生素C亚铁盐
维生素C乙基醚
维生素 C 磷酸酯镁
维生素 C
维他命C磷酸镁盐
维他命C杂质
纯绿青霉酸
粘氯酸酐
粘氯酸酐
粘氯酸酐
粘康酸内酯
粉青霉酸酐
穿心莲丁素
硫酰胺,(3-氰基-5,6,7,8-四氢-4H-环庚三烯并[b]噻吩并-2-基)-(9CI)
白头翁素
甲基[(2S,3R)-2-乙氧基-3,6-二氢-2H-吡喃-3-基]乙酸酯
甲基7-氧杂双环[2.2.1]庚-2,5-二烯-2-羧酸酯
甲基5-甲基-4,5-二氢-3-呋喃羧酸酯
甲基4-氰基-2,5-二氢-3-呋喃羧酸酯
甲基4-氧代四氢-2-呋喃羧酸酯
甲基4,5-二氢-2-呋喃羧酸酯
甲基3-甲基-2,3-二氢-3-呋喃羧酸酯