5-(phenoxymethyl)-3-(p-tolyl)oxazolidin-2-one | 5255-84-5
中文名称
——
中文别名
——
英文名称
5-(phenoxymethyl)-3-(p-tolyl)oxazolidin-2-one
英文别名
5-phenoxymethyl-3-p-tolyl-oxazolidin-2-one;N-(4-Tolyl)-5-phenoxymethyl-oxazolidon-(2);3-p-Tolyl-5-phenoxymethyl-oxazolidon-(2);3-p-Tolyl-5-phenoxymethyl-2-oxazolidon;5-Phenoxymethyl-3-(p-tolyl)-2-oxazolidone;3-(4-methylphenyl)-5-(phenoxymethyl)-1,3-oxazolidin-2-one
CAS
5255-84-5
化学式
C17H17NO3
mdl
——
分子量
283.327
InChiKey
OKLOMSSBTQAMCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:149-151 °C
-
沸点:428.8±24.0 °C(Predicted)
-
密度:1.194±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:21
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.24
-
拓扑面积:38.8
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为产物:参考文献:名称:异氰酸酯和环氧化物合成 2-恶唑烷酮的双功能相转移催化剂摘要:合成了一系列双功能相转移催化剂 (PTC),以催化异氰酸酯和环氧化物的 [3 + 2] 偶联反应,以良好至高产率(高达 92% 产率)提供 2-恶唑烷酮,使用 PhCl 作为溶剂12 小时内达到 100 °C。这些双功能 PTC 很容易由市售的叔伯二胺和异氰酸酯(或异硫氰酸酯、单方方酰胺)通过两个简单的步骤制备,具有良好的模块化和高效率(2.5 mol% 催化剂负载)。季铵盐中心和催化剂中的氢键供体基团与底物的协同作用对这种原子经济反应至关重要。DOI:10.1039/d0ra00693a
文献信息
-
Microwave-Assisted Electrostatically Enhanced Phenol-Catalyzed Synthesis of Oxazolidinones作者:Ali Rostami、Amirhossein Ebrahimi、Nader Sakhaee、Farhad Golmohammadi、Ahmed Al-HarrasiDOI:10.1021/acs.joc.1c01686日期:2022.1.7one-component organocatalyst for the atom-economic transformation of epoxides to oxazolidinones under microwave irradiation. Integrating a positively charged center into phenols over a modular one-step preparation gives rise to a bifunctional system with improved acidity and activity, competent in rapid assembly of epoxides and isocyanates under microwave irradiation in a short reaction time (20–60 min)静电增强的苯酚被用作一种简单、可持续和有效的单组分有机催化剂,用于在微波照射下将环氧化物原子经济地转化为恶唑烷酮。通过模块化的一步制备将带正电荷的中心整合到酚类中,产生了一个双功能体系,具有改善的酸度和活性,能够在微波照射下在短反应时间(20-60 分钟)内快速组装环氧化物和异氰酸酯。仔细评估了各种带正电荷的苯酚和苯胺的功效,并检查了催化剂负载、温度和亲核试剂种类等几个因素对催化反应性的影响。在整洁的条件下,利用这一单组分催化平台,可在数分钟内从各种芳基和烷基取代的环氧化物和异氰酸酯制备 40 多个恶唑烷酮实例,实现了高达 96% 的收率和高选择性。进行了 DFT 计算以实现不同催化途径的反应势垒,以提供机理理解并证实了其中提出的同时环氧化物开环和异氰酸酯掺入的实验结果。
-
A Multicomponent Approach to Oxazolidinone Synthesis Catalyzed by Rare‐Earth Metal Amides作者:Meixia Zhou、Xizhou Zheng、Yaorong Wang、Dan Yuan、Yingming YaoDOI:10.1002/cctc.201900221日期:2019.12.5Three‐component reaction of epoxides, amines, and dimethyl carbonate catalyzed by rare‐earth metal amides has been developed to synthesize oxazolidinones. 47 examples of 3,5‐disubstituted oxazolidinones were prepared in 13–97 % yields. This is a simple and most practical method which employs easily available substrates and catalysts, and is applicable to a wide range of aromatic and aliphatic amines
-
2-Oxazolidones from Glycidyl Ether Reactions with Acid Amides作者:Yoshio Iwakura、Shin-ichi IzawaDOI:10.1246/bcsj.39.2490日期:1966.11The reaction between acid amides and aryl glycidyl ethers was carried out using tertiary amine as the catalyst. 2-Oxazolidone derivatives were obtained by the reaction of trichloroacetanilide or trifluoroacetanilide with aryl glycidyl ether. Acyl migration occurred in the reaction of acetanilide with phenyl glycidyl ether.
-
An Approach to Synthesis of 3-Aryl-2-Oxazolidinones and In Situ`Click` Assembly of 1,2,3-Triazole Oxazolidinones作者:Xingxian Zhang、Cheng Li、Wei Chen、Xiang WuDOI:10.2174/157017810791112414日期:2010.4.1A facile and efficient addition of isocyanates with epoxides in the presence of MgI2 etherate was reported in good yields. The corresponding 2-oxazolidinone could be easily converted into 1,2,3-triazole-oxazolidinone by click reaction in excellent yield.
-
一种五元含氧杂环化合物的合成方法
表征谱图
-
氢谱1HNMR
-
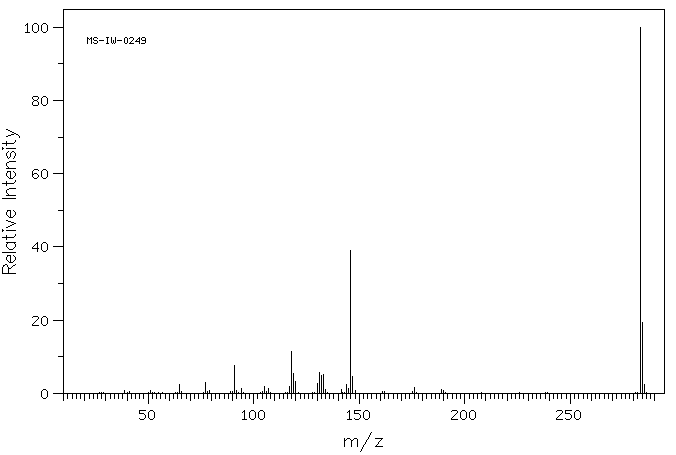
质谱MS
-
碳谱13CNMR
-
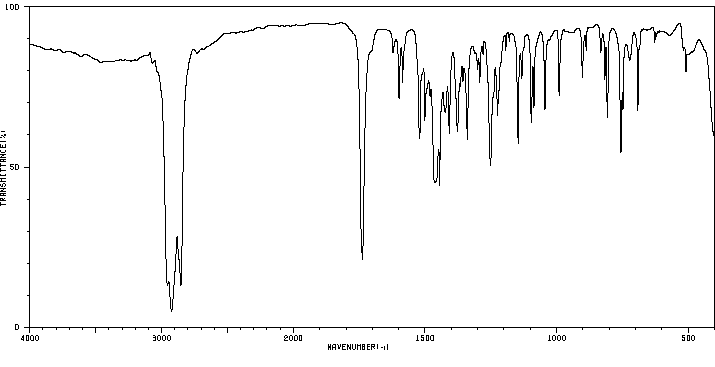
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯