sphaeropsidin C methyl ester
中文名称
——
中文别名
——
英文名称
sphaeropsidin C methyl ester
英文别名
methyl (4aR,4bR,7R,10aS)-7-ethenyl-4b-hydroxy-1,1,7-trimethyl-9-oxo-3,4,5,6,10,10a-hexahydro-2H-phenanthrene-4a-carboxylate
CAS
——
化学式
C21H30O4
mdl
——
分子量
346.467
InChiKey
BYODNWRRBVBGDG-LRGNLBRXSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:25
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:63.6
-
氢给体数:1
-
氢受体数:4
反应信息
-
作为产物:描述:sphaeropsidin C 、 碘甲烷 在 potassium carbonate 作用下, 以 丙酮 为溶剂, 反应 1.0h, 以5 mg的产率得到sphaeropsidin C methyl ester参考文献:名称:Smardaesidins A–G、Isopimarane 和 20-nor-Isopimarane Diterpenoids 来自 Smardaea sp.,一种苔藓 Ceratodon purpureus 的真菌内生菌 (1)摘要:五种新的异海松二萜、smardaesidins A–E ( 1 – 5 ) 和两种新的 20- nor- isopimarane 二萜、smardaesidins F ( 6 ) 和 G ( 7 ),以及sphaeropsidins A ( 8 ) 和 C–F ( 10 – 13 ) ) 是从内生真菌菌株Smardaea sp.中分离出来的。AZ0432,存在于苔藓Ceratodon purpureus 的活光合组织中。其中,作为不可分离的异构体混合物获得了smardaesidins B( 2 )和C( 3 )。sphaeropsidin A ( 8 ) 的化学还原提供了 sphaeropsidin B ( 9 )),而8 的催化氢化产生 7 - O -15,16-四氢球藻素 A ( 14 ) 及其新衍生物 7-羟基-6-氧代异海松- 7-en-20-油酸 ( 15 )。sphaeropsidinDOI:10.1021/np2000864
文献信息
-
<i>In Vitro</i> Antibacterial Activity of Sphaeropsidins and Chemical Derivatives toward <i>Xanthomonas oryzae</i> pv. <i>oryzae</i>, the Causal Agent of Rice Bacterial Blight作者:Antonio Evidente、Vittorio Venturi、Marco Masi、Giuliano Degrassi、Alessio Cimmino、Lucia Maddau、Anna AndolfiDOI:10.1021/np200625m日期:2011.12.27Sphaeropsidin A, the main phytotoxin produced by Diplodia cupressi, as well as the two natural analogues sphaeropsidins B and C and 14 derivatives obtained by chemical modifications were assayed for antibacterial activity against Xanthomonas oryzae pv. oryzae, Pseudomonas fuscovaginae, and Burkholderia glumae, the causal agents of severe bacterial rice diseases. The results showed a strong and specific activity of sphaeropsidin A against X. oryzae pv. oryzae, while no activity was observed against the other two pathogens. The results of structure-activity relationship studies showed that structural features important to impart this antibacterial activity are the presence of the C-7 carbonyl group and the hemiketalic lactone functionality. The C-13 vinyl group, the double bond of ring C, and/or the tertiary C-9 hydroxy group, as well as the pimarane arrangement of the tricylic carbon skeleton, were also important for the antibacterial activity. These findings may be useful in designing novel compounds for practical applications in agriculture.
-
Studies on structure–activity relationship of sphaeropsidins A–F, phytotoxins produced by Sphaeropsis sapinea f. sp. cupressi作者:Lorenzo Sparapano、Giovanni Bruno、Olga Fierro、Antonio EvidenteDOI:10.1016/j.phytochem.2003.11.006日期:2004.1Six forms of sphaeropsidins (SA-SF), three- and tetra-cyclic unrearranged pimarane diterpenes produced by Sphaeropsis sapinea f. sp. cupressi, as well as eight derivatives obtained by chemical modification of SA-SC, were assayed for their bioactivity. The effect of each compound on plants which are host or non-host of the pathogen was investigated. Activity on some plant pathogenic fungi was also tested. Some structure-activity relationships have been identified for both phytotoxic and antifungal activity. It appears that the integrity of the tricyclic pimarane system, the preservation of the double bond C(8)-C(14), the tertiary hydroxyl group at C-9, the vinyl group at C-13, and the carboxylic group at C-10 as well as the integrity of the A-ring provide these molecules with non selective phytotoxic and antimycotic activity. (C) 2003 Elsevier Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
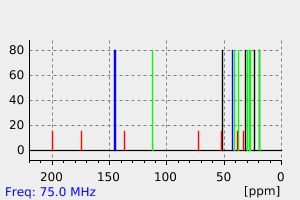
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩