2',6'-dihydroxychalcone | 25515-44-0
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,6-二羟基苯乙酮 2,6-Dihydroxyacetophenone 699-83-2 C8H8O3 152.15
反应信息
-
作为反应物:描述:参考文献:名称:2',6'-二羟基查尔酮及其衍生物环化的动力学和机理摘要:报道了pH–速率曲线,表明在水中环化成2',6'-二羟基查尔酮(1)及其4-甲氧基(2),3,4-二甲氧基(3),3,4,5-的5-羟基黄酮三甲氧基(4),2,4,6-三甲氧基(5),4-氯(6)和3,4,4'-三甲氧基(8)衍生物。至于先前研究的2',6'-二羟基-4,4'-二甲氧基查尔酮(7)的速率系数是为中性查尔酮的酸催化环化,中性,单阴离子和二阴离子查尔酮的单分子环化以及碱催化的反向开环反应而确定的。2',6'-二羟基查耳酮单阴离子的环化速度比2'-羟基-6'-甲氧基查耳酮的单阴离子的环化速度快40倍(10),并且据估计比2',4'-二羟基查耳酮的反应性单阴离子快约十倍。这些是6'-OH基团提高单阴离子环化速率的首次计算。该作用仅很小,并且被认为主要是由于氢键合烯醇化氧而使酮化的过渡态稳定。还讨论了查耳酮单阴离子之间的其他反应性差异。报告了单阴离子环化(1),(2)和(4)–(6)DOI:10.1039/p29890001623
-
作为产物:描述:2'-hydroxy-6'-tetrahydropyranyloxyacetophenone 在 盐酸 、 氢氧化钾 作用下, 以 甲醇 、 乙醇 、 水 为溶剂, 反应 6.08h, 生成 2',6'-dihydroxychalcone参考文献:名称:Miles, Christopher O.; Main, Lyndsay; Nicholson, Brian K., Australian Journal of Chemistry, 1989, vol. 42, # 7, p. 1103 - 1114摘要:DOI:
文献信息
-
2′,5′-Dihydroxychalcone as a Potent Chemical Mediator and Cyclooxygenase Inhibitor作者:Chun-Nan Lin、Tai-Hua Lee、Mei-Feng Hsu、Jih-Pyang Wang、Feng-Nien Ko、Che-Ming TengDOI:10.1111/j.2042-7158.1997.tb06837.x日期:2011.4.12
Abstract Eleven chalcone derivatives have been tested for their inhibitory effects on platelet aggregation in rabbit platelet suspension and the activation of mast cells and neutrophils.
Arachidonic acid-induced platelet aggregation was potently inhibited by almost all the compounds and some also had a potent inhibitory effect on collagen-induced platelet aggregation and cyclooxygenase. Some hydroxychalcone derivatives showed strong inhibitory effects on the release of β-glucuronidase and lysozyme, and on superoxide formation by rat neutrophils stimulated with the peptide fMet-Leu-Phe (fMLP). We found that the anti-inflammatory effect of 2′,5′-dihydroxychalcone was greater than that of trifluoperazine. 2′,5′-Dihydroxy and 2′,3,4,4′-tetrahydroxyl chalcones, even at low concentration (50 μm), tested in platelet-rich plasma from man almost completely inhibited secondary aggregation induced by adrenaline.
These results suggest that the anti-platelet effects of the chalcones are mainly a result of inhibition of thromboxane formation.
摘要:对十一种查尔酮衍生物进行了测试,以评估它们对兔血小板悬液中血小板聚集、肥大细胞和中性粒细胞激活的抑制作用。花生四烯酸诱导的血小板聚集几乎被所有化合物强烈抑制,有些化合物对胶原诱导的血小板聚集和环氧化酶也有强烈的抑制作用。一些羟基查尔酮衍生物显示出对β-葡萄糖苷酸酶和溶菌酶释放,以及对受到fMet-Leu-Phe(fMLP)肽激活的大鼠中性粒细胞产生的超氧化物的强烈抑制作用。我们发现,2′,5′-二羟基查尔酮的抗炎效果大于三氟她嗪。即使在低浓度(50 μm)下,在人体富含血小板的血浆中,2′,5′-二羟基和2′,3,4,4′-四羟基查尔酮几乎完全抑制了由肾上腺素诱导的继发性聚集。这些结果表明,查尔酮的抗血小板作用主要是通过抑制血栓素形成实现的。 -
Conversion of 2′-hydroxychalcones to flavanones catalyzed by cobalt Schiff base complex作者:Kazushige Maruyama、Kimihiro Tamanaka、Akira Nishinaga、Akira Inada、Tsutomu NakanishiDOI:10.1016/s0040-4039(00)99344-4日期:1989.1Co(salpr) catalyzes the conversion of 2′-hydroxychalcones to flavanones in methanol under oxygen. Base catalysis by Co(salpr) (OH) produced in situ is responsible for the reaction, which is found to proceed reversibly.
-
<i>In Vitro</i>and<i>in Vivo</i>Antischistosomal Activities of Chalcones作者:Vinícius R. D. Pereira、Ismael J. Alves Junior、Lígia S. da Silveira、Reinaldo B. Geraldo、Priscila de F. Pinto、Fernanda S. Teixeira、Maria C. Salvadori、Marcos P. Silva、Lara A. Alves、Priscila V. S. Z. Capriles、Ayla das C. Almeida、Elaine S. Coimbra、Pedro L. S. Pinto、Mara R. C. Couri、Josué de Moraes、Ademar A. Da Silva FilhoDOI:10.1002/cbdv.201800398日期:——in vitro incubation with chalcones 1 and 3. In a mouse model of schistosomiasis, the oral treatment (400 mg/kg) with chalcone 1 or 3 significantly caused a total worm burden reduction in mice. Chalcone 1 showed significant inhibition of the S. mansoni ATP diphosphohydrolase activity, which was corroborated by molecular docking studies. The results suggested that chalcones could be explored as lead
-
Potential application value of hydroxychalcones based on isoliquiritigenin in agricultural plant diseases
-
Synthesis and evaluation of 2′,4′,6′-trihydroxychalcones as a new class of tyrosinase inhibitors作者:Nishida Jun、Gao Hong、Kawabata JunDOI:10.1016/j.bmc.2007.01.017日期:2007.3In this study, we synthesized a series of hydroxychalcones and examined their tyrosinase inhibitory activity. The results showed that 2',4',6'-trihydroxychalcone (1), 2,2',3,4',6'-pentahydroxychalcone (4), 2',3,4,4,5,6'-hexahydroxychalcone (5), 2',4',6'-trihydroxy- 3,4-dimethoxychalcone (9) and 2,2,4,4',6'-pentahydroxychalcone (15) exhibited high inhibitory effects on tyrosinase with respect to L-tyrosine as a substrate. By the structure activity relationship study, it was suggested that the 2',4',6'-trihydroxyl substructure in the chalcone skeleton were efficacious for the inhibition of tyrosinase activity. And also, the catechol structure on B-ring of chalcones was not advantageous for the inhibitory potency. Furthermore, 15 (IC50 = 1 mu M) was found to show the highest activity out of a set of 15 hydroxychalcones, even better than both 2,2',4,4'-tetrahydroxychalcone (13, IC50 = 5 mu M) and kojic acid (16, IC50 = 12 mu M), which were known as potent tyrosinase inhibitors. Kinetic study revealed that 15 acts as a competitive inhibitor of tyrosinase with K-i value of 3.1 mu M. (c) 2007 Elsevier Ltd. All rights reserved.
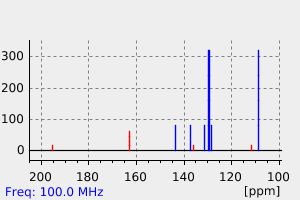
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息