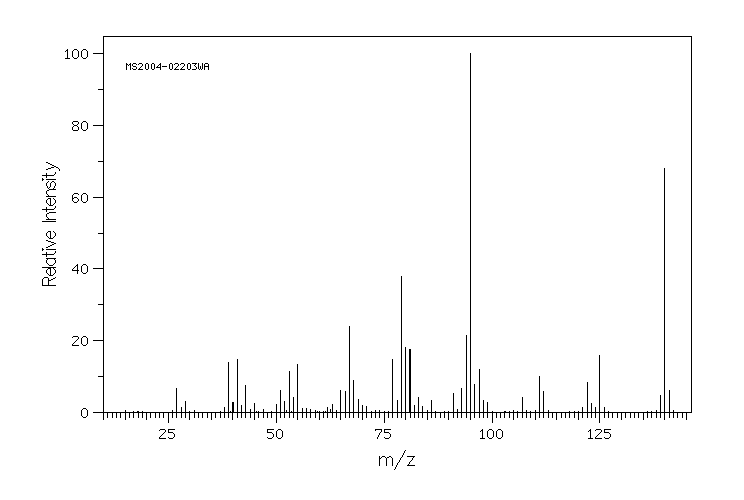
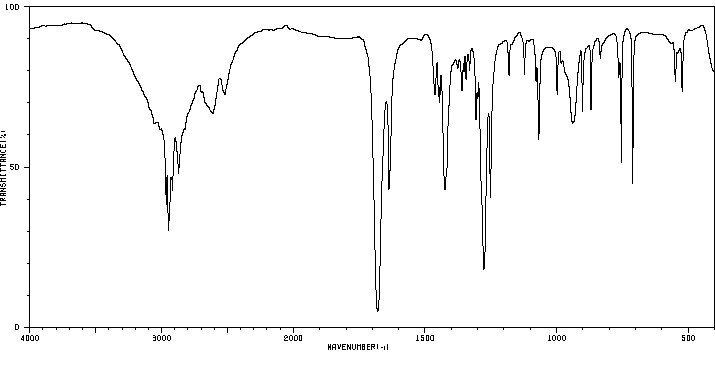
6-甲基-环己-1-烯羧酸 | 5726-56-7
中文名称
6-甲基-环己-1-烯羧酸
中文别名
——
英文名称
6-methylcyclohex-1-ene-1-carboxylic acid
英文别名
6-methyl-cyclohex-1-enecarboxylic acid;6-Methyl-cyclohex-1-encarbonsaeure;2-Methyl-Δ6-tetrahydrobenzoesaeure;Δ6-Tetrahydro-o-toluylsaeure;6-Methylcyclohex-1-enecarboxylic acid;6-methylcyclohexene-1-carboxylic acid
CAS
5726-56-7
化学式
C8H12O2
mdl
MFCD19228164
分子量
140.182
InChiKey
DCXQIBDJDGKXQL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.625
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2916209090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 6-Methyl-cyclohex-1-en-carbonsaeure-tert-butylester 5933-65-3 C12H20O2 196.29 —— 3a,4,5,6-tetrahydro-3H-isobenzofuran-1-one 503273-45-8 C8H10O2 138.166 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 6-Methyl-3-oxo-cyclohex-1-enecarboxylic acid methyl ester 69413-86-1 C9H12O3 168.192 (9ci)-6-甲基-1-环己烯-1-羰酰氯 6-methyl-cyclohex-1-enecarbonyl chloride 72622-71-0 C8H11ClO 158.628
反应信息
-
作为反应物:描述:6-甲基-环己-1-烯羧酸 在 permanganate(VII) ion 作用下, 生成 2-甲基己二酸参考文献:名称:Mazza; di Mase, Gazzetta Chimica Italiana, 1927, vol. 57, p. 308摘要:DOI:
-
作为产物:描述:参考文献:名称:合成中不饱和羧酸的二烯二醇。通过不饱和羧酸的串联Michael-Dieckmann脱羧环化反应合成环己酮和多环酮。摘要:通过将无环和脂环式不饱和羧酸的二烯二醇锂串联加入相同或其他不饱和羧酸的锂盐中来制备取代的2-环己烯酮4至7和六氢萘二烯酮和六氢茚并酮13至18。DOI:10.1016/s0040-4020(01)86973-8
文献信息
-
Cobalt- and Nickel-Catalyzed Carboxylation of Alkenyl and Sterically Hindered Aryl Triflates Utilizing CO<sub>2</sub>作者:Keisuke Nogi、Tetsuaki Fujihara、Jun Terao、Yasushi TsujiDOI:10.1021/acs.joc.5b02307日期:2015.11.20cobalt-catalyzed reductive carboxylation reaction of alkenyl trifluoromethanesulfonates (triflates) has been developed. By employing Mn powder as a reducing reagent under 1 atm pressure of CO2 at room temperature, diverse alkenyl triflates can be converted to the corresponding α,β-unsaturated carboxylic acids. Moreover, the carboxylation of sterically hindered aryl triflates proceeds smoothly in the presence of
-
Activation of Aryl and Vinyl Triflates by Palladium and Electron Transfer – Electrosynthesis of Aromatic and α,β-Unsaturated Carboxylic Acids from Carbon Dioxide作者:Anny Jutand、Serge NégriDOI:10.1002/(sici)1099-0690(199809)1998:9<1811::aid-ejoc1811>3.0.co;2-v日期:1998.9The electrochemical reduction of aryl and vinyl triflates in the presence of CO2 and a catalytic amount of palladium results in the formation of aromatic and α,β-unsaturated carboxylic acids. Aryl and vinyl triflates usually undergo palladium-catalysed cross-coupling reactions with nucleophiles. Their reactivity has been reversed in the presence of an electron source, so that they react with electrophiles
-
[1+4] Cycloaddition of vinyl isocyanates with isocyanides. Construction of functionally elaborate pyrrolinone derivatives作者:James H. Rigby、Maher Qabar、Gulzar Ahmed、Robert C. HughesDOI:10.1016/s0040-4020(01)80551-2日期:1993.1Reaction of alkyl isocyanides with vinyl isocyanates affords highly functionalized pyrrolinone and hydroindolone products via a novel [1+4] cyclization process.
-
Palladium-Catalyzed Carboxylation of Vinyl Triflates. Electrosynthesis of α,β-Unsaturated Carboxylic Acids作者:Anny Jutand、Serge NégriDOI:10.1055/s-1997-3241日期:1997.6The palladium-catalyzed electrocarboxylation of vinyl triflates affords α,β-unsaturated carboxylic acids. The reactivity of vinyl triflates has been reversed in the presence of an electron source, since they now react with electrophiles such as CO2.
-
Functionalization of<i>trans</i>-Decalin. III. A Stereospecific Preparation of Vicinal<i>cis</i>Two Methyl Groups of Eremophilane Skeleton, Leading to<i>dl</i>-Dehydrofukinone作者:Sigeru Torii、Tsutomu Inokuchi、Tetsuo YamafujiDOI:10.1246/bcsj.52.2640日期:1979.9A procedure for the preparation of dl-dehydrofukinone (1), an eremophilane type sesquiterpene, from the diene adducts (3), prepared from the reaction of 4-methyl-3-methoxycarbonyl-2-cyclohexen-1-one with butadiene, is described. Acetalization-reduction (LiAlH4) of 3 followed by treatment of the corresponding mesylate with base provided 5,6-cis-5-methyltricyclo[4.4.1.01,6]undec-8-en-2-one (7) in 69% overall yields. Reductive cleavage of 7 with lithium metal afforded trans-4β,4aβ-dimethyl-Δ6,7-octalin-1-one (8), bearing a set of vicinal cis two methyl groups on the C-4 and C-4a carbons in an 83% yield. Functionalization of the double bond of 8 involves (1) reduction of carbonyl group and following tetrahydropyranylation, (2) epoxidation followed by regiospecific reduction of the oxirane ring at the C-6 position, and (3) subsequent oxidation of the hydroxyl group, giving trans-4aβ,5β-dimethyl-8β-tetrahydropyranyloxydecalin-2-one (14b) in good yields. The conversion of 14b into the desired 1 was achieved smoothly by (1) hydrolysis of tetrahydropyranyl ether, (2) pyrolysis of its mesylate, and (3) subsequent aldol reaction with acetone followed with dehydration and isomerization of the double bond.本文描述了一种从 4-甲基-3-甲氧羰基-2-环己烯-1-酮与丁二烯反应制备的二烯加合物(3)中制备 dl-脱氢紫杉酮(1)(一种 eremophilane 型倍半萜)的方法。先对 3 进行乙缩醛化还原(LiAlH4),然后用碱处理相应的甲磺酸盐,最后得到 5,6-顺式-5-甲基三环[4.4.1.01,6]十一碳-8-烯-2-酮(7),总产率为 69%。用金属锂对 7 进行还原裂解,得到了反式-4β,4aβ-二甲基-Δ6,7-辛烯-1-酮(8),该化合物在 C-4 和 C-4a 碳上带有一组邻接的顺式两个甲基,收率为 83%。8 双键的官能化过程包括:(1) 还原羰基,然后进行四氢吡喃化;(2) 环氧化,然后在 C-6 位进行环氧乙烷环的区域特异性还原;(3) 随后氧化羟基,得到反式-4aβ,5β-二甲基-8β-四氢吡喃氧基萘-2-酮(14b),收率很高。通过 (1) 四氢吡喃基醚的水解,(2) 其甲磺酸盐的热解,(3) 随后与丙酮的醛醇反应,以及双键的脱水和异构化,可以顺利地将 14b 转化为所需的 1。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸