6-甲氧基-1-甲基-9H-吡啶并(3,4-b)吲哚 | 3589-72-8
中文名称
6-甲氧基-1-甲基-9H-吡啶并(3,4-b)吲哚
中文别名
6-甲氧基哈尔满
英文名称
6-methoxyharman
英文别名
6-Methoxy-1-methyl-9H-pyrido[3,4-b]indole 6-methoxy-1-methyl-9H-pyrido[3,4-b]indole;6-methoxy-1-methyl-9H-pyrido[3,4-b]indole;12-Methoxy-6-methyl-β-carboline;6-methoxyharmane;isoharmine
CAS
3589-72-8
化学式
C13H12N2O
mdl
MFCD01544241
分子量
212.251
InChiKey
XYYVPBBISSKKQB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:275°C (dec.)
-
沸点:421.4±40.0 °C(Predicted)
-
密度:1.252±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:16
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.153
-
拓扑面积:37.9
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2933990090
-
储存条件:室温
SDS
Version 1.2
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name 6-METHOXYHARMAN
2 - Hazards Identification
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
6-METHOXYHARMAN 3589-72-8 None None
Formula C13H12N2O
Molecular Weight 212,3000 AMU
Synonyms Coharmine * Isoharmine * 6-Methoxyharman *
6-Methoxyharmane *
6-Methoxy-1-methyl-9H-pyrido(3,4-b)indole
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If breathing becomes difficult,
call a physician.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
SIGMA www.molbase.com
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear protective equipment.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
STORAGE
Store at 2-8°C
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Mechanical exhaust required.
PERSONAL PROTECTIVE EQUIPMENT
Special Protective Measures: Wear appropriate government approved
respirator, chemical-resistant gloves, safety goggles, other
protective clothing.
9 - Physical and Chemical Properties
Appearance Physical State: Solid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
SIGMA www.molbase.com
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, carbon dioxide,
and nitrogen oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
RTECS NUMBER: UV0168000
ACUTE TOXICITY
LD50
Intraperitoneal
Mouse
650 MG/KG
Remarks: Kidney, Ureter, Bladder:Urine volume increased.
ROUTE OF EXPOSURE
Multiple Routes: May be harmful by inhalation, ingestion, or
skin absorption. May cause irritation.
CONDITIONS AGGRAVATED BY EXPOSURE
The toxicological properties have not been thoroughly
investigated.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and
scrubber. Observe all federal, state, and local environmental
regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
SIGMA www.molbase.com
Non-hazardous for air transport.
15 - Regulatory Information
Caution: Substance not yet fully tested (EU).
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
SIGMA www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name 6-METHOXYHARMAN
2 - Hazards Identification
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
6-METHOXYHARMAN 3589-72-8 None None
Formula C13H12N2O
Molecular Weight 212,3000 AMU
Synonyms Coharmine * Isoharmine * 6-Methoxyharman *
6-Methoxyharmane *
6-Methoxy-1-methyl-9H-pyrido(3,4-b)indole
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If breathing becomes difficult,
call a physician.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
SIGMA www.molbase.com
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear protective equipment.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
STORAGE
Store at 2-8°C
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Mechanical exhaust required.
PERSONAL PROTECTIVE EQUIPMENT
Special Protective Measures: Wear appropriate government approved
respirator, chemical-resistant gloves, safety goggles, other
protective clothing.
9 - Physical and Chemical Properties
Appearance Physical State: Solid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
SIGMA www.molbase.com
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, carbon dioxide,
and nitrogen oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
RTECS NUMBER: UV0168000
ACUTE TOXICITY
LD50
Intraperitoneal
Mouse
650 MG/KG
Remarks: Kidney, Ureter, Bladder:Urine volume increased.
ROUTE OF EXPOSURE
Multiple Routes: May be harmful by inhalation, ingestion, or
skin absorption. May cause irritation.
CONDITIONS AGGRAVATED BY EXPOSURE
The toxicological properties have not been thoroughly
investigated.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and
scrubber. Observe all federal, state, and local environmental
regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
SIGMA www.molbase.com
Non-hazardous for air transport.
15 - Regulatory Information
Caution: Substance not yet fully tested (EU).
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
SIGMA www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-甲基-9h-吡啶并[3,4-b]吲哚-6-醇 6-Hydroxyharman 67767-19-5 C12H10N2O 198.224 —— 6-methoxy-1,9-dimethyl-9H-β-carboline 22574-29-4 C14H14N2O 226.278 —— 1,9-dimethyl-9H-pyrido[3,4-b]indol-6-ol 916330-37-5 C13H12N2O 212.251 —— 4-(6-methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)butan-1-amine 1342261-06-6 C17H21N3O 283.373
反应信息
-
作为反应物:描述:6-甲氧基-1-甲基-9H-吡啶并(3,4-b)吲哚 在 sodium hydride 、 一水合肼 作用下, 以 甲醇 、 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 生成 4-(6-methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)butan-1-amine参考文献:名称:Structure–activity relationship study of beta-carboline derivatives as haspin kinase inhibitors摘要:Haspin is a serine/threonine kinase that phosphorylates Thr-3 of histone H3 in mitosis that has emerged as a possible cancer therapeutic target. High throughput screening of approximately 140,000 compounds identified the beta-carbolines harmine and harmol as moderately potent haspin kinase inhibitors. Based on information obtained from a structure-activity relationship study previously conducted for an acridine series of haspin inhibitors in conjunction with in silico docking using a recently disclosed crystal structure of the kinase, harmine analogs were designed that resulted in significantly increased haspin kinase inhibitory potency. The harmine derivatives also demonstrated less activity towards DYRK2 compared to the acridine series. In vitro mouse liver microsome stability and kinase profiling of a representative member of the harmine series (42, LDN-211898) are also presented. (C) 2012 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2012.01.028
-
作为产物:描述:褪黑素 在 氧气 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 三氯氧磷 作用下, 以 二甲基亚砜 、 乙酸乙酯 、 乙腈 为溶剂, 反应 18.0h, 生成 6-甲氧基-1-甲基-9H-吡啶并(3,4-b)吲哚参考文献:名称:具有与阿尔茨海默病相关的治疗作用的心塑性 DYRK1A 抑制剂摘要:Tau蛋白病、神经元萎缩和心理障碍是阿尔茨海默病等神经退行性疾病的标志,目前缺乏能够纠正这些问题的有效临床治疗方法。为了解决这些未满足的需求,我们采用合理的药物设计,将DYRK1A抑制剂和isoDMT的药效团相结合,开发出心生DYRK1A抑制剂。通过这种方法,我们发现了一种非致幻性化合物,能够促进皮质神经元生长并抑制 tau 蛋白过度磷酸化,同时还具有减轻痴呆症的生物和心理症状的潜力。总之,我们的结果表明,DYRK1A 和精神质体原药效团的杂交代表了一种有前途的策略,用于识别可能解决痴呆症的认知以及行为和心理症状的化合物。DOI:10.1021/acs.jmedchem.3c01696
文献信息
-
A new approach to 1-substituted β-carbolines and isoquinolines utilizing tributyl[(Z)-2-ethoxyvinyl]stannane as a C-3,C-4 building block作者:Alexandra Kamlah、Florian Lirk、Franz BracherDOI:10.1016/j.tet.2015.12.049日期:2016.2from readily available indole-2-carboxylic acids, 1-substituted β-carbolines (among them the alkaloids harmane and isoharmine) are readily obtained via the corresponding 2-acylindoles, bromination at C-3, followed by a one-pot Stille cross-coupling with tributyl[(Z)-2-ethoxyvinyl]stannane, and ring closure with ammonium acetate. 1-Substituted isoquinolines are available in an analogous manner starting
-
Further investigation of harmicines as novel antiplasmodial agents: Synthesis, structure-activity relationship and insight into the mechanism of action作者:Marina Marinović、Goran Poje、Ivana Perković、Diana Fontinha、Miguel Prudêncio、Jana Held、Lais Pessanha de Carvalho、Tana Tandarić、Robert Vianello、Zrinka RajićDOI:10.1016/j.ejmech.2021.113687日期:2021.11demonstrated that harmicines, hybrid compounds composed from β-carboline alkaloid harmine and cinnamic acid derivatives, linked via either triazole or amide bond, exert significant antiplasmodial activity. In this paper, we report synthesis, antiplasmodial activity and cytotoxicity of expanded series of novel triazole- and amide-type harmicines. Structure-activity relationship analysis revealed that amide-type疟原虫对目前批准的疗法的耐药性的上升促使发现和开发新的有效药物。以前我们已经证明,由 β-咔啉生物碱和肉桂酸衍生物组成的杂化化合物,通过三唑或酰胺键连接,具有显着的抗疟原虫活性。在本文中,我们报告了一系列新型三唑类和酰胺类药物的合成、抗疟原虫活性和细胞毒性。构效关系分析表明,在 β-咔啉核心的 N-9 处制备的酰胺类药物27对恶性疟原虫的红细胞阶段和伯氏疟原虫的肝阶段均表现出优异的效力. 值得注意的是,harmicine 27a,m -(三氟甲基)肉桂酸衍生物,表现出最有利的选择性指数(SI = 1105)。分子动力学模拟揭示了恶性疟原虫热休克蛋白 90的 ATP 结合位点作为药物结合位点,证实了有害物质 N-9 取代的有用性,并确定了有利的 N–H … π 相互作用,涉及 Lys45 和芳香族苯基单元附加的肉桂酸片段对于增强的生物活性至关重要。因此,这些化合物被确定为在寻找新型、更有效的
-
Microwave-Assisted Synthesis of Tetrahydro-β-carbolines and β-Carbolines作者:Scott Eagon、Marc O. AndersonDOI:10.1002/ejoc.201301580日期:2014.3chromatography. This method tolerates a wide range of functionality and can be performed on milligram to gram scales. A subsequent microwave-mediated aromatization of the synthesized tetrahydro-β-carbolines to β-carbolines was also developed utilizing catalytic Pd/C. The aromatization is complete in 60 min or less with most substrates requiring minimal purification.
-
Structural development of canthin-5,6-dione moiety as a fluorescent dye and its application to novel fluorescent sensors作者:Hidetomo Yokoo、Ayumi Ohsaki、Hiroyuki Kagechika、Tomoya HiranoDOI:10.1016/j.tet.2016.08.014日期:2016.9Among the synthesized compounds, 5 showed an ‘OFF-ON-OFF’-type fluorescence change with increase of pH, and therefore served as a novel fluorescent sensor for a specific range of pH. Our findings suggest that canthin-5,6-dione should be useful as a fluorophore moiety for fluorescent labeling of biomolecules and for development of fluorescent probes and sensors.Canthin-5,6-dione是荧光天然产物amarastelline A和nigakinone的常见结构。在这里,我们合成了各种衍生物,并研究了它们的荧光特性。澄清了在N 3-,4-和10-位上的取代基的作用。N 3-取代通过调节canthin-5,6-dione和5-hydroxy-6-one形式之间的互变异构作用来影响溶剂依赖性荧光变化。10-取代也与N 3-取代一起影响了荧光,特别是在水溶液中。在合成的化合物中,有5种随pH的升高显示“ OFF-ON-OFF”型荧光变化,因此可作为特定pH范围的新型荧光传感器。我们的发现表明,canthin-5,6-dione应该用作荧光团部分,用于生物分子的荧光标记以及开发荧光探针和传感器。
-
Design and synthesis of harmiquins, harmine and chloroquine hybrids as potent antiplasmodial agents作者:Goran Poje、Lais Pessanha de Carvalho、Jana Held、Diana Moita、Miguel Prudêncio、Ivana Perković、Tana Tandarić、Robert Vianello、Zrinka RajićDOI:10.1016/j.ejmech.2022.114408日期:2022.8significantly higher activity than CQ against the resistant Plasmodium strains and had a very high selectivity index (4450). Harmiquins may act through the inhibition of heme polymerization and binding to the ATP binding site of the PfHsp90, which would explain their increased activity against the CQ-resistant Plasmodium strains. These results establish harmiquins as valuable antiplasmodial hits for future疟疾仍然是世界范围内的主要健康问题之一。缺乏有效的疫苗以及疟原虫对已批准的抗疟药物的耐药性增加,需要开发能够有效预防和/或治疗这种疾病的新型抗疟原虫药物。 Harmiquins 代表在一个分子中结合具有不同抗疟原虫活性机制的两个部分的杂种,即已知抑制血红素聚合的氯喹 (CQ) 支架和能够与恶性疟原虫热休克蛋白 90 ( Pf ) 结合的 β-咔啉环。热休克蛋白90)。在这里,我们介绍了它们的合成、生物活性的评价和潜在的作用机制。合成的杂化物在所使用的接头类型(三唑环或酰胺键)以及在harmine的β-咔啉核心上的取代位置不同。针对疟原虫的红细胞阶段评估了harmiquins的抗疟原虫活性生命周期,并在 HepG2 细胞上测试了它们的细胞毒作用。结果表明,harmiquins 对 CQ 敏感(Pf 3D7)和 CQ 抗性(Pf Dd2、Pf K1 和Pf 7G8)均具有显着的活性。恶性疟原虫
表征谱图
-
氢谱1HNMR
-
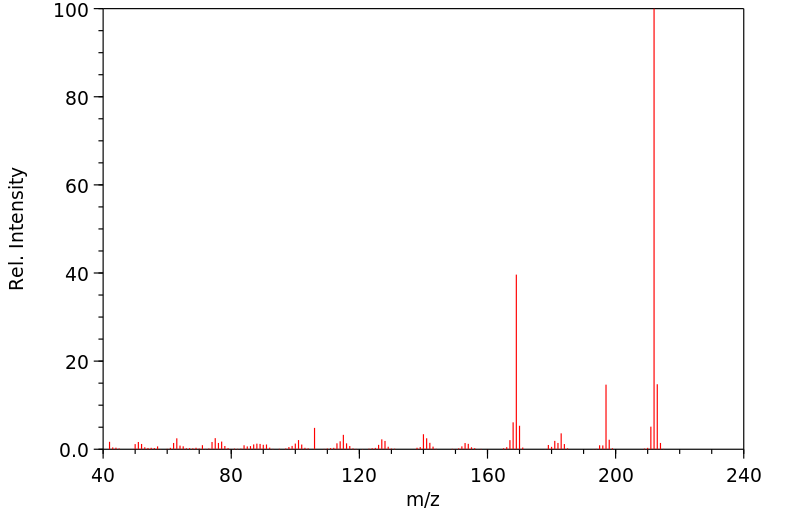
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
鲁贝替定
骆驼蓬酚盐酸盐
骆驼蓬碱-d3
骆驼蓬灵
银柴胡胺B
酒渣碱
苦林双碱乙
苦木西碱 J
苦木西碱 I
苦木碱 A
色氨酸EP杂质E
肉叶云香碱
短苔草碱
盐酸骆驼蓬灵
盐酸哈尔酚水合物
盐酸哈尔酚
盐酸去氢骆驼蓬碱
甲基1-甲基-2,3,4,9-四氢-1H-beta-咔啉-1-羧酸酯
甲基1-[5-(羟甲基)-2-呋喃基]-9H-β-咔啉-3-羧酸酯
甲基(2S,3S,4S)-3-(羟基甲基)-2-甲基-4-[(9-甲基-9H-beta-咔啉-1-基)甲基]-3,4-二氢-2H-吡喃-5-羧酸酯
淡紫醌霉素
氢溴酸加兰它敏
川芎哚
外消旋1-三氯甲基-1,2,3,4-四氢-beta-咔啉
四氢骆驼蓬碱
哈尔酚硫酸盐
哈尔酚
哈尔满碱-D3
哈尔满碱-13C2,15N
哈尔满碱
哈尔满盐酸盐
含苦木西碱A
去甲骆驼蓬碱
去氢苦木碱
八角枫叶碱
他达那非杂质D
他达那非杂质B
他达拉非标准品HCL
他达拉非杂质A
他达拉非杂质92
他达拉非杂质8
他达拉非杂质20
他达拉非杂质13
他达拉非中间体酯水解杂质
二乙氨基前他达拉非
乙酮,1-(7-溴-9H-吡啶并[3,4-b]吲哚-1-基)-2-苯基-
乙基1-吡啶-3-基-2,3,4,9-四氢-1H-β-咔啉-3-羧酸酯
乙基1-(2-乙氧基-2-氧代乙基)-2,3,4,9-四氢-1H-beta-咔啉-1-羧酸酯盐酸盐(1:1)
Γ-咔啉
beta-咔啉-1-丙酸