7-氧代壬酸 | 20356-92-7
中文名称
7-氧代壬酸
中文别名
——
英文名称
7-oxononanoic acid
英文别名
7-Oxo-nonansaeure;7-Oxo-nonanoic acid
CAS
20356-92-7
化学式
C9H16O3
mdl
MFCD01320161
分子量
172.224
InChiKey
BMDUEIZQNLDNCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:12
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.777
-
拓扑面积:54.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2918300090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Huenig et al., Chemische Berichte, 1958, vol. 91, p. 129,133摘要:DOI:
-
作为产物:参考文献:名称:Blaise; Koehler, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1909, vol. 148, p. 490摘要:DOI:
文献信息
-
Heterocycle Derivatives As Histone Deacetylase (Hdac) Inhibitors申请人:Attenni Barbara公开号:US20090048228A1公开(公告)日:2009-02-19The present invention relates to compounds of formula I: or pharmaceutically acceptable salts or tautomers thereof, which are inhibitors of histone deacetylase (HDAC). The compounds of the present invention are useful for treating cellular proliferative diseases, including cancer. Further, the compounds of the present invention are useful for treating neurodegenerative diseases, schizophrenia and stroke among other diseases.
-
[EN] HALOALKENE COMPOUNDS, PROCESS FOR THEIR PRODUCTION AND PESTICIDES CONTAINING THEM<br/>[FR] HALOALCENES, PROCEDE DE PRODUCTION D'HALOALCENES ET PESTICIDES CONTENANT CES HALOALCENES申请人:ISHIHARA SANGYO KAISHA公开号:WO2004052872A1公开(公告)日:2004-06-24ABSTRACT A novel haloalkene compound represented by the formula (I), useful as an active ingredient of a pesticide, is presented: wherein each of X1 and X2 is halogen, Y is a hydrogen atom, halogen, alkyl or the like, n is from 0 to 5, A is an oxygen atom or a sulfur atom, G is a hydrogen atom, alkyl, acyl or the like, and Q is a 5- to 12-membered heterocyclic group (the heterocyclic group may be substituted) containing an optional hetero atom selected from an oxygen atom, a sulfur atom and a nitrogen atom, alkyl which may be substituted by W, alkenyl which may be substituted by W, alkynyl which may be substituted by W, or cycloalkyl which may be substituted by W, wherein W is halogen, alkoxy, alkylthio, hydroxyl, cyano, nitro or phenyl which may be substituted.
-
[EN] HALOALKENE COMPOUNDS, PROCESS FOR THEIR PRODUCTION AND PESTICIDES CONTAINING THEM<br/>[FR] COMPOSES D'HALOALCENE, PROCEDE DE PRODUCTION ET PESTICIDES LES CONTENANT申请人:ISHIHARA SANGYO KAISHA公开号:WO2005063018A1公开(公告)日:2005-07-14Present invention relates to a haloalkene compound of the formula (I)or its salt: wherein each of X1 and X2 is halogen; Y is alkyl, haloalkyl or phenyl; n is 0 to 5; L is -C(=B)Q, -C(=B)B’Q, -C(=B)N(D)Q, -N(D)Q, -N(D)C(=B)Q, -N(D)C(=B)B’Q, -N(D)SO2Q, -N=CHQ, -N=C(Q)2, -SO2Q, -SO2N(D)Q or alkyl substituted by J, G is a hydrogen atom, alkyl, haloalkyl, phenylalkyl, alkenyl, haloalkenyl, phenylalkenyl, alkynyl, haloalkynyl, phenylalkynyl, -C(=B)Q, -C(=B)B’Q, -C(=B)N(G’)Q, -SQ, -SO2Q, -SN(G’)Q, -SN(G’)C(=B)B’Q or -SO2N(G’)Q; A is an oxygen atom or a sulfur atom.本发明涉及一种具有以下式(I)或其盐的卤烯化合物:其中X1和X2中的每一个都是卤素;Y是烷基、卤代烷基或苯基;n为0至5;L为-C(=B)Q、-C(=B)B’Q、-C(=B)N(D)Q、-N(D)Q、-N(D)C(=B)Q、-N(D)C(=B)B’Q、-N(D)SO2Q、-N=CHQ、-N=C(Q)2、-SO2Q、-SO2N(D)Q或被J取代的烷基;G是氢原子、烷基、卤代烷基、苯基烷基、烯基、卤代烯基、苯基烯基、炔基、卤代炔基、苯基炔基、-C(=B)Q、-C(=B)B’Q、-C(=B)N(G’)Q、-SQ、-SO2Q、-SN(G’)Q、-SN(G’)C(=B)B’Q或-SO2N(G’)Q;A是氧原子或硫原子。
-
Alkaline Cleavage of Unsymmetrical β-Diketones. Ring Opening of Acylcylohexanones to Form ε-Acyl Caproic Acids<sup>1</sup>作者:Charles R. Hauser、Frederic W. Swamer、Betty I. RinglerDOI:10.1021/ja01192a016日期:1948.12
-
The influence of the lipophilicity of 7-oxoacyl-l-alanyl-d-isoglutamines on their immunorestoration activity in vivo作者:M Sollner、S Pečar、A ŠtalcDOI:10.1016/s0223-5234(97)89858-3日期:1996.1In search for immunologically active dipeptides, a series of new N-(7-oxoacyl)-L-alanyl-D-isoglutamines has been synthesized where lipophilicity was varied by changing the 7-oxoacyl residue from 7-oxooctanoyl to 7-oxotetradecanoyl. The immunological properties of the compounds were examined in an in vivo immunorestoration test. It was found that lipophilicity had a significant influence on the potency of the compounds tested. Compounds between N-(7-oxodecanoyl)-L-alanyl-D-isoglutamine and N-(7-oxotetradecanoyl)-L-alanyl-D-isoglutamine showed biphasic influence on efficacy. The most active compound found was 7-oxododecanoyl-L-alanyl-D-isoglutamine, which also has an activity comparable to that of azimexone.
表征谱图
-
氢谱1HNMR
-
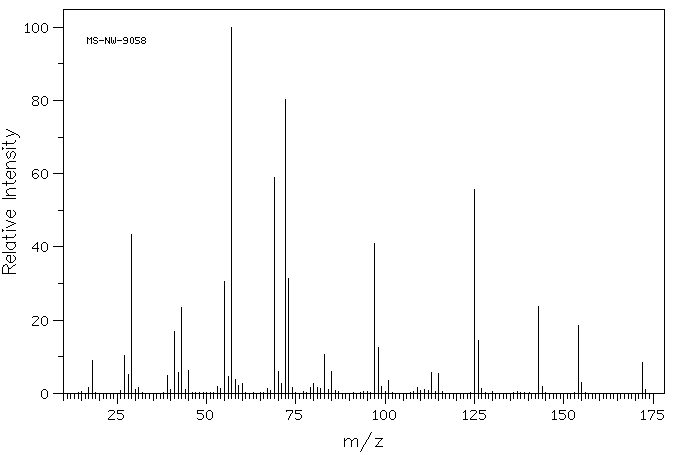
质谱MS
-
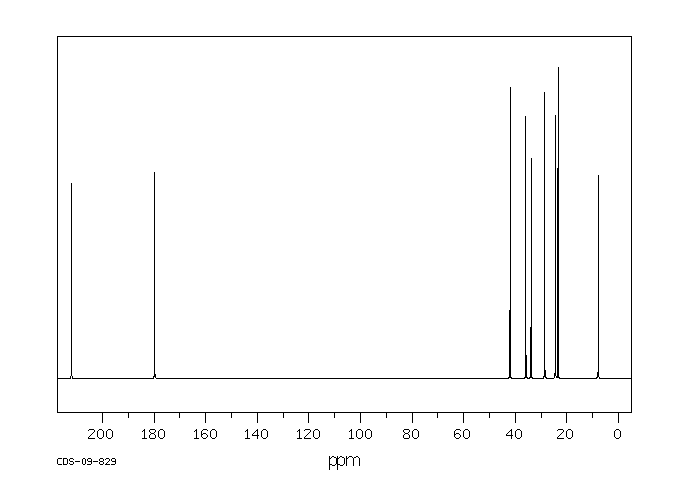
碳谱13CNMR
-
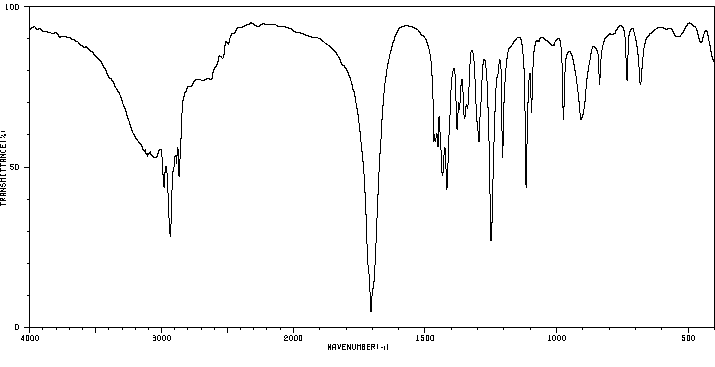
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯