苯基 2-氨基苯甲酸 | 10268-69-6
物质功能分类
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:70°C
-
沸点:382.7±25.0 °C(Predicted)
-
密度:1.217±0.06 g/cm3(Predicted)
-
溶解度:Insoluble in water, poorly soluble in cold alcohol. Soluble in most perfume oils.
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:52.3
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
海关编码:2922439000
-
储存条件:室温且干燥
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— phenyl 2-azidobenzoate 16714-22-0 C13H9N3O2 239.233 —— phenyl 2-nitrobenzoate 31042-59-8 C13H9NO4 243.219 邻氨基苯甲酸 anthranilic acid 118-92-3 C7H7NO2 137.138 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— phenyl 2-azidobenzoate 16714-22-0 C13H9N3O2 239.233 邻氨基苯甲酸 anthranilic acid 118-92-3 C7H7NO2 137.138
反应信息
-
作为反应物:描述:参考文献:名称:Algarra, Felipe; Miranda, Miguel A., Heterocycles, 1993, vol. 36, # 10, p. 2335 - 2344摘要:DOI:
-
作为产物:描述:参考文献:名称:Rule; Bretscher, Journal of the Chemical Society, 1927, p. 926摘要:DOI:
文献信息
-
Intramolecular General Base Catalyzed Ester Hydrolysis. The Hydrolysis of 2-Aminobenzoate Esters作者:Thomas H. Fife、Randhir Singh、Ramesh BembiDOI:10.1021/jo0103017日期:2002.5.1with the phenyl ester is 10(5)-fold. Intramolecular general base catalyzed reactions are assessed in respect to their relative advantages and disadvantages in enzyme catalysis. A general base catalyzed reaction can be more rapid at low pH than a nucleophilic reaction that has a marked dependence on pH and the leaving group.已经获得了在50°C下于H(2)O中水解2-氨基苯甲酸的三氟乙基,苯基和对硝基苯基酯的速率常数。拟一级反应速率常数k(obsd)与pH无关,与pH 8至pH 4(胺基共轭酸的pK(a))无关。2-氨基苯甲酸酯在不依赖pH的反应中以相似的速率常数水解,在D(2)O中,这些水反应比H(2)O中的反应慢约2倍。最可能的机制涉及相邻胺基团在分子内的一般碱催化作用。与相应的对位取代的酯或苯甲酸酯的不依赖pH的水解相比,不依赖pH的反应的速率提高了50-100倍。与氢氧根离子催化的反应相比,用苯酯在pH 4下k(obsd)的增强是10(5)倍。就其在酶催化中的相对优点和缺点,评估了分子内一般碱催化的反应。一般的碱催化反应在低pH条件下比对pH和离去基团有明显依赖性的亲核反应更快。
-
Substituent Effects on the Base-Catalysed Hydrolysis of Phenyl Esters of ortho-Substituted Benzoic Acids作者:Ingrid Bauerová、Miroslav LudwigDOI:10.1135/cccc20010770日期:——
Fourteen model phenyl esters of 2-substituted benzoic acids were synthesised. Structures and purity of model compounds were confirmed by 1H and 13C NMR spectroscopy, as well as by HPLC and elemental analysis. Kinetics of base-catalysed hydrolysis of model phenyl esters occurring by the BAc2 mechanism were measured by UV spectrophotometry in 50% (v/v) aqueous dimethyl sulfoxide solutions at 25 °C under pseudo-first-order reaction conditions (
c (NaOH) = 0.001-1.0 mol l-1). Linear relation betweenJ -E and logk obs with the slope close to unity was found for all model compounds. Neither one-parameter nor multiparameter Hammett-type description of variability of experimental data obtained for phenyl esters of 2-substituted benzoic acids was found. Two groups (conjugating and non-conjugating) were created by division ofortho -substituents inortho -position using the AISE theory, based on their interaction with the reaction centre. -
Metal‐Free Synthesis of Anthranils by PhIO Mediated Heterocyclization of <i>ortho</i> ‐Carbonyl Anilines作者:Alankrita Garia、Jatin Grover、Nidhi JainDOI:10.1002/ejoc.202100756日期:2021.8.6A metal-free synthesis of anthranils from ortho-carbonyl anilines using PhIO as a sole reagent under ambient conditions is described. No external additives are required, the reaction has broad substrate scope and delivers anthranils in excellent yields via oxidative heterocyclization.
-
Derivative of saccharide and physiologically active agent containing the申请人:Kureha Kagaku Kogyo Kabushiki Kaisha公开号:US04372948A1公开(公告)日:1983-02-08The novel derivatives of saccharide obtained by bringing a saccharide into reaction with an ester of aminobenzoic acid, an aminobenzoic acid amide, an aminophenylacetic acid or an ester thereof have various physiological activities.
-
Kinetic Study of Hydrolysis of Benzoates. Part XXV. Ortho Substituent Effect in Alkaline Hydrolysis of Phenyl Esters of Substituted Benzoic Acids in Water作者:Vilve Nummert、Mare Piirsalu、Vahur Mäemets、Ilmar KoppelDOI:10.1135/cccc20060107日期:——
The second-order rate constants
k 2 for alkaline hydrolysis of phenyl esters ofmeta -,para - andortho -substituted benzoic acids, X-C6H4CO2C6H5 (X = H, 3-Cl, 3-NO2, 3-CH3, 4-NO2, 4-Cl, 4-F, 4-CH3, 4-OCH3, 4-NH2, 2-NO2, 2-CN, 2-F, 2-Cl, 2-Br, 2-I, 2-CH3, 2-OCH3, 2-CF3, 2-NH2), and of substituted phenyl esters of benzoic acid, C6H5CO2C6H4-X (X = 2-I, 2-CF3, 2-C(CH3)3, 4-Cl, 4-CH3, 4-OCH3, 4-NH2), have been measured spectrophotometrically in water at 25 °C. The substituent effect in alkaline hydrolysis of phenyl esters ofpara -substituted benzoic acids, similar to that for ethyl esters ofpara -substituted benzoic acids, was found to be precisely described by the Hammett relationship (ρ = 1.7 in water). The logk value for alkaline hydrolysis of phenyl and ethyl esters ofmeta -,para - andortho -substituted benzoic acids, X-C6H4CO2R, was nicely correlated with logk m,p,ortho = logk o + (ρ)m,pσ + (ρI)orthoσI + (ρ°R)orthoσ°R + δorthoE sB where σ, σI, σ°R are the Hammett polar, Taft inductive and Taft resonance (σ°R = σ° - σI) substituent constants, respectively.E sB is the steric scale forortho substituents calculated on the basis of the logk values for the acid hydrolysis ofortho - substituted phenyl benzoates in water owing to theortho substituent in the phenyl of phenyl benzoates. In water, the main factors responsible for changes in theortho substituent effect in alkaline hydrolysis of phenyl and ethyl esters ofortho -substituted benzoic acids, X-C6H4CO2R, were found to be the inductive and steric factors while the role of the resonance term was negligible ((ρ°R)ortho ca. 0.3). In alkaline hydrolysis of substituted benzoates in neat water, theortho inductive effect appeared to be 1.5 times and steric influence 2.7 times higher than the corresponding influences from theortho position in the phenyl of phenyl benzoates. The contributions of the steric effects in alkaline hydrolysis of esters ofortho -substituted benzoic acids was found to be approximately the same as in acid hydrolysis of esters ofortho -substituted benzoic and acid esterification ofortho -substituted benzoic acids.对苯酸酯碱水解的二阶速率常数 k2,对邻、对、间取代苯甲酸的苯酯 X-C6H4CO2C6H5(X = H、3-Cl、3-NO2、3-CH3、4- 、4-Cl、4-F、4- 、4-O 、4-NH2、2- 、2-CN、2-F、2-Cl、2-Br、2-I、2- 、2-O 、2-CF3、2-NH2)以及苯甲酸苯酯的取代苯酯 C6H5CO2C6H4-X(X = 2-I、2- 、2-C( )3、4-Cl、4- 、4-O 、4-NH2)在25°C的水中进行了分光光度测定。对邻取代苯甲酸的苯酯碱水解的取代基效应,类似于对邻取代苯甲酸的乙酯,被发现可以精确地由 Hammett 关系描述(ρ = 1.7 在水中)。对邻、对、间取代苯甲酸的苯酯和乙酯 X-C6H4CO2R 的碱水解的 log k 值与 log km,p,ortho = log ko + (ρ)m,pσ + (ρI)orthoσI + (ρ°R)orthoσ°R + δorthoE sB 相关联,其中σ、σI、σ°R 是 Hammett 极性、Taft 归纳和Taft 共振(σ°R = σ° - σI)取代基常数,E sB 是基于对苯甲酸苯酯的苯基中取代基的酸水解 log k 值计算的对位取代基的立体规模。在水中,对苯酸酯碱水解的对取代基效应的主要因素被发现为归纳和立体因素,而共振项的作用微乎其微((ρ°R)ortho 约为0.3)。在水中,对苯酸酯碱水解的对位取代基的立体影响似乎比对苯甲酸苯酯的苯基中的对位影响高出1.5倍,而归纳影响则高出2.7倍。对苯酸酯碱水解的对位取代基的立体效应的贡献与对苯酸酯碱水解的酸和对位取代基酯化的酸酯化大致相同。
表征谱图
-
氢谱1HNMR
-
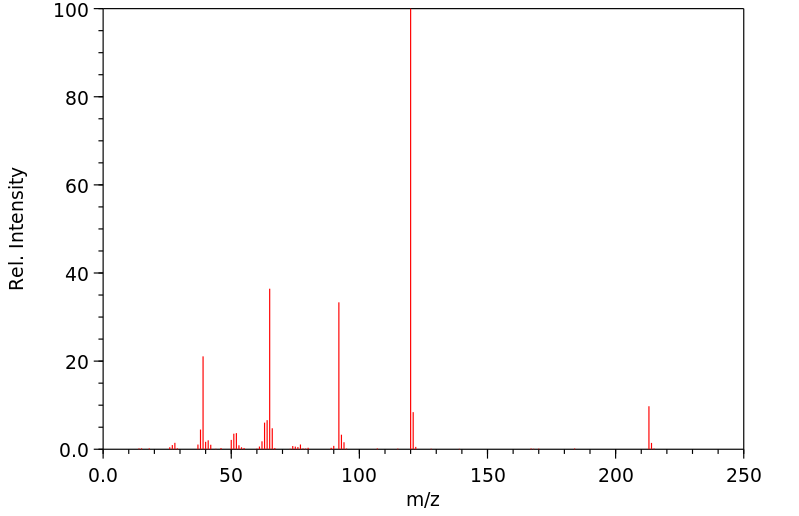
质谱MS
-
碳谱13CNMR
-
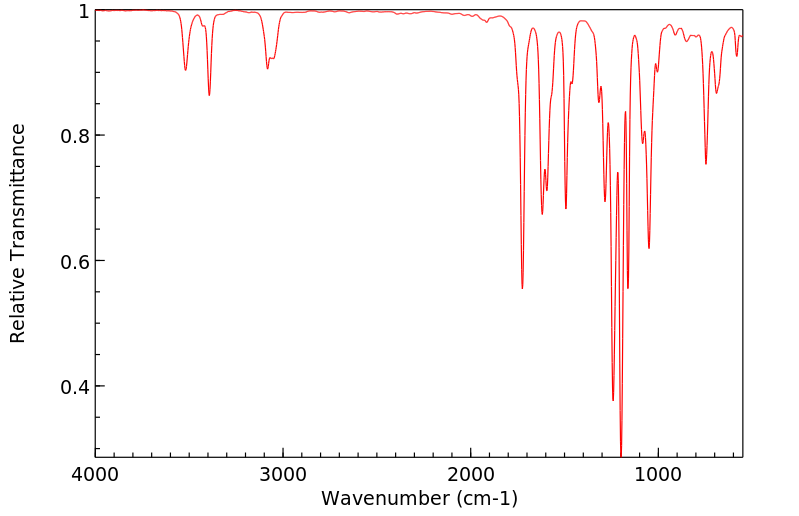
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息