苯基-3-(2-噻唑基)-2-硫脲 | 14901-16-7
中文名称
苯基-3-(2-噻唑基)-2-硫脲
中文别名
1-苯基-3-(2-噻唑基)-2-硫脲
英文名称
1-phenyl-3-(thiazol-2-yl)thiourea
英文别名
1-phenyl-3-(1,3-thiazol-2-yl)thiourea;1-phenyl-3-(2-thiazolyl)-2-thiourea;1-phenyl-3-(thiazole-2-yl)thiourea;N-phenyl-N'-(2-thiazolyl) thiourea;N-phenyl-N'-(thiazol-2-yl)thiourea;1-phenyl-3-(thiazolyl)-2-thiourea
CAS
14901-16-7
化学式
C10H9N3S2
mdl
MFCD00005321
分子量
235.334
InChiKey
GCZZOZBWAZHCAN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:183-185 °C (dec.)(lit.)
-
密度:1.3479 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:97.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
安全说明:S26,S39
-
危险类别码:R22,R41,R37/38
-
WGK Germany:3
-
RTECS号:YU1398000
-
海关编码:2934100090
SDS
| Name: | 1-Phenyl-3-(2-Thiazolyl)-2-Thiourea 97+% Material Safety Data Sheet |
| Synonym: | Thiourea, N-Phenyl-N'-2-Thiazolyl- |
| CAS: | 14901-16-7 |
Synonym:Thiourea, N-Phenyl-N'-2-Thiazolyl-
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 14901-16-7 | 1-Phenyl-3-(2-Thiazolyl)-2-Thiourea | 97+ | 238-970-4 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 14901-16-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 183.00 - 185.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H9N3S2
Molecular Weight: 235.32
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, sulfur oxides (SOx), including sulfur oxide and sulfur dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 14901-16-7: YU1398000 LD50/LC50:
Not available.
Carcinogenicity:
1-Phenyl-3-(2-Thiazolyl)-2-Thiourea - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 14901-16-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 14901-16-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 14901-16-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:苯基-3-(2-噻唑基)-2-硫脲 在 potassium carbonate 作用下, 以 异丙醇 、 丙酮 为溶剂, 生成 N-phenyl-N'-(thiazol-2-yl)cyanoguanidine参考文献:名称:微波辅助合成N,N'-二芳基氰基胍。摘要:[反应:见正文]描述了在微波辅助条件下从其相应的硫脲合成N,N'-二芳基氰基胍的温和,高效且高产方法。合成了一系列同时具有给电子和吸电子取代基的氰基胍。通过使用极性溶剂以及适度的温度来促进反应。DOI:10.1021/ol050728q
-
作为产物:描述:生成 四氰基乙烯 、 苯基-3-(2-噻唑基)-2-硫脲参考文献:名称:Mohamed; Hassan; Ibrahim, Pharmazie, 1992, vol. 47, # 8, p. 592 - 595摘要:DOI:
文献信息
-
Efficient construction of C–C bonds from aryl halides/aryl esters with arylboronic acids catalysed by palladium(II) thiourea complexes作者:Manikandan Thimma Sambamoorthy、Ramesh Rengan、Malecki Jan GrzegorzDOI:10.1002/aoc.5181日期:2019.11Interestingly, the palladium(II) thiourea complexes showed the highest catalytic activity with 0.1 mol% catalyst loading in Suzuki–Miyaura cross‐coupling reactions utilizing a range of aryl bromides/unactivated aryl chlorides with arylboronic acids as coupling partners in aqueous–organic media. Syntheses of diaryl ketones using aryl esters and arylboronic acids as coupling partners were also achieved with low已成功地合成了一组新的包含苯基(噻唑基)硫脲配体的钯(II)配合物,并借助分析和光谱(IR,UV-可见光和NMR)方法对其进行了表征。单晶X射线衍射法证实了新钯配合物中硫脲配体的N ^ S配位模式具有N ^ S配位模式的扭曲方平面几何结构。有趣的是,钯(II)硫脲配合物在铃木-宫浦交叉偶联反应中显示出最高的催化活性,催化剂负载量为0.1摩尔%,利用一系列芳基溴化物/未活化的芳基氯化物与芳基硼酸作为水性有机介质中的偶合伙伴。使用芳基酯和芳基硼酸作为偶合伙伴合成二芳基酮也可以在20小时内以较低的催化剂负载量实现。我们的催化剂的潜力通过其广泛的底物范围,低的催化剂负载量和高的分离产率得到了证明。此外,还研究了关键参数如溶剂,碱,温度和催化剂用量的影响。
-
Novel Thiazole Derivatives of Medicinal Potential: Synthesis and Modeling作者:Nour E. A. Abdel-Sattar、Abeer M. El-Naggar、M. S. A. Abdel-MottalebDOI:10.1155/2017/4102796日期:——This paper reports on the synthesis of new thiazole derivatives that could be profitably exploited in medical treatment of tumors. Molecular electronic structures have been modeled within density function theory (DFT) framework. Reactivity indices obtained from the frontier orbital energies as well as electrostatic potential energy maps are discussed and correlated with the molecular structure. X-ray
-
Nickel(II)‐Catalyzed Selective <i>(E)</i> ‐Olefination of Methyl Heteroarenes Using Benzyl Alcohols via Acceptorless Dehydrogenative Coupling Reaction作者:Gunasekaran Balamurugan、Rengan RameshDOI:10.1002/cctc.202101455日期:2022.1.21characterized with FT-IR, NMR, HRMS and single crystal X-ray method. The new complexes were tuned as effective homogeneous catalysts for synthesis of selective (E)-olefins from the reaction of methyl heteroarenes and benzyl alcohols via greener ADC methodology. The earth abundant nickel complexes performed well with 4 mol% of catalyst loading at 110 °C for 35 h, resulting in good to excellent yields (40–93 %)
-
Antimicrobial and Anti-biofilm Activity of Thiourea Derivatives Incorporating a 2-Aminothiazole Scaffold作者:Joanna Stefanska、Grażyna Nowicka、Marta Struga、Daniel Szulczyk、Anna Eugenia Koziol、Ewa Augustynowicz-Kopec、Agnieszka Napiorkowska、Anna Bielenica、Wojciech Filipowski、Anna Filipowska、Aleksandra Drzewiecka、Gabriele Giliberti、Silvia Madeddu、Stefano Boi、Paolo La Colla、Giuseppina SannaDOI:10.1248/cpb.c14-00837日期:——A series of new thiourea derivatives of 1,3-thiazole have been synthesized. All obtained compounds were tested in vitro against a number of microorganisms, including Gram-positive cocci, Gram-negative rods and Candida albicans. Compounds were also tested for their in vitro tuberculostatic activity against the Mycobacterium tuberculosis H37Rv strain, as well as two ‘wild’ strains isolated from tuberculosis patients. Compounds 3 and 9 showed significant inhibition against Gram-positive cocci (standard strains and hospital strain). The range of MIC values is 2–32 µg/mL. Products 3 and 9 effectively inhibited the biofilm formation of both methicillin-resistant and standard strains of S. epidermidis. The halogen atom, especially at the 3rd position of the phenyl group, is significantly important for this antimicrobial activity. Moreover, all obtained compounds resulted in cytotoxicity and antiviral activity on a large set of DNA and RNA viruses, including Human Immunodeficiency Virus type 1 (HIV-1) and other several important human pathogens. Compound 4 showed activity against HIV-1 and Coxsackievirus type B5. Seven compounds resulted in cytotoxicity against MT-4 cells (CC50<10 µM).我们合成了一系列新的 1,3-噻唑硫脲衍生物。对所有获得的化合物都进行了抗多种微生物(包括革兰氏阳性球菌、革兰氏阴性杆菌和白色念珠菌)的体外测试。此外,还测试了化合物对结核分枝杆菌 H37Rv 菌株以及从结核病患者体内分离出的两种 "野生 "菌株的体外抗结核活性。化合物 3 和 9 对革兰氏阳性球菌(标准菌株和医院菌株)有明显的抑制作用。MIC 值范围为 2-32 µg/mL。产品 3 和 9 能有效抑制耐甲氧西林表皮葡萄球菌和标准菌株的生物膜形成。卤素原子,尤其是位于苯基第 3 位的卤素原子,对这种抗菌活性非常重要。此外,所有获得的化合物都对大量 DNA 和 RNA 病毒具有细胞毒性和抗病毒活性,包括人类免疫缺陷病毒 1 型(HIV-1)和其他几种重要的人类病原体。化合物 4 对 HIV-1 和 B5 型柯萨奇病毒具有活性。7 种化合物对 MT-4 细胞具有细胞毒性(CC50<10 µM)。
-
One-pot synthesis of thiazolo[3,4-a]quinoxalines and the related heterocyclic systems using 4-hydroxy-4-alkoxycarbonyl-3,5-diaryl-2-aryliminothia(selena)zolidines as versatile reagents作者:Vakhid A. Mamedov、Nataliya A. Zhukova、Alsu A. Balandina、Sergey V. Kharlamov、Tat'yana N. Beschastnova、Il'dar Kh. Rizvanov、Shamil K. LatypovDOI:10.1016/j.tet.2012.06.084日期:2012.9An efficient and versatile one-step method for the synthesis of thiazolo[3,4-a]quinoxalines and related new heterocyclic systems have been developed on the basis of a new strategy for the construction of the pyrazine ring system. The key step of the process involves the cascade annulation of the iminothiazolopyrazine system to benzene in the reaction of 4-hydroxy-3,5-diaryl-2-phenyliminothiazolidines
表征谱图
-
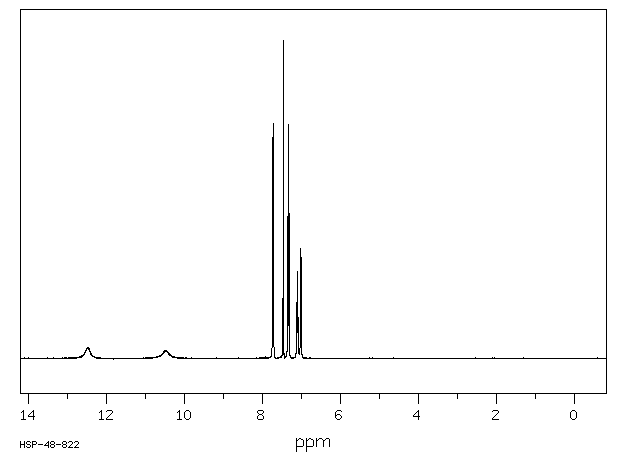
氢谱1HNMR
-
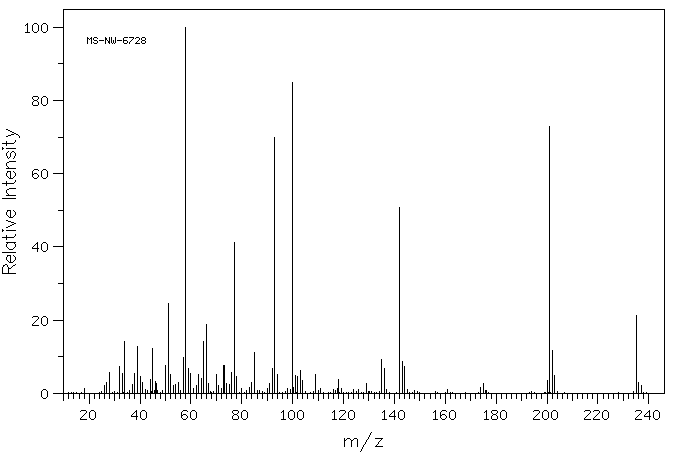
质谱MS
-
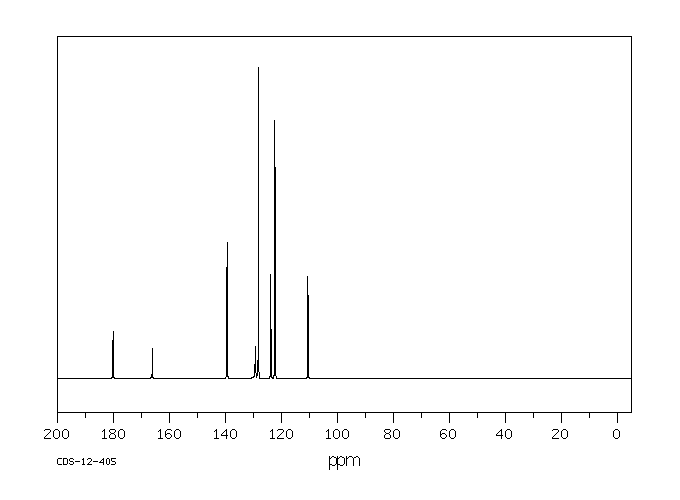
碳谱13CNMR
-
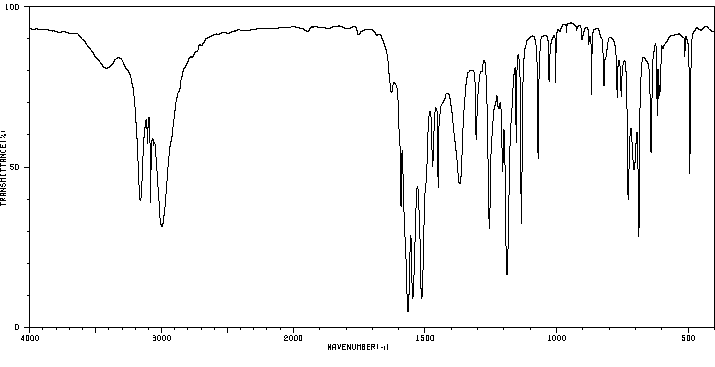
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫