9-硝基菲 | 954-46-1
中文名称
9-硝基菲
中文别名
——
英文名称
9-nitrophenanthrene
英文别名
——
CAS
954-46-1
化学式
C14H9NO2
mdl
——
分子量
223.231
InChiKey
QTTCNQHPKFAYEZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:116-117 °C
-
沸点:413.3±14.0 °C(Predicted)
-
密度:1.316±0.06 g/cm3(Predicted)
-
保留指数:364.5
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:17
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2904209090
-
储存条件:请将容器密封储存于阴凉干燥处。
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Meisenheimer, Justus Liebigs Annalen der Chemie, 1907, vol. 355, p. 309摘要:DOI:
-
作为产物:参考文献:名称:亚硝酸铋和亚硫酰氯选择性硝化芳香族化合物摘要:已发现碱式硝酸铋/亚硫酰氯是一种有效的试剂组合,可用于在二氯甲烷中硝化多种芳香族化合物。尤其是酚类,通过控制化学计量,很容易与试剂一起单硝化和二硝化,DOI:10.3390/80700593
文献信息
-
<i>ipso</i>-Nitration of Arylboronic Acids with Bismuth Nitrate and Perdisulfate作者:Srimanta Manna、Soham Maity、Sujoy Rana、Soumitra Agasti、Debabrata MaitiDOI:10.1021/ol300325t日期:2012.4.6An efficient and one pot synthetic method of ipso-nitration of arylboronic acids has been developed. The high efficiency, general applicability, and broader substrate scope including heterocycles and functional groups make this method advantageous. Due to its simplicity, we expect to find application of this method in synthesis.的有效和一锅合成法本位的芳基硼酸-nitration已经研制成功。高效,普遍适用性以及包括杂环和官能团在内的更大的底物范围使该方法具有优势。由于其简单性,我们期望在合成中找到该方法的应用。
-
Formation of Arenesvia Diallylarenes: Strategic Utilization of Suzuki–Miyaura Cross-Coupling, Claisen Rearrangement and Ring-Closing Metathesis作者:Sambasivarao Kotha、Vrajesh R. Shah、Kalyaneswar MandalDOI:10.1002/adsc.200600469日期:2007.5.7benzoannulation are reported. The first strategy is based on the Suzuki–Miyaura cross-coupling reaction. To this end, various ortho-diallylbenzene derivatives were prepared from the corrresponding diiodo derivatives by an allylation strategy using an allylboronate as coupling partner. These diallyl derivatives were subjected to a ring-closing metathesis (RCM) and one-pot dichlorodicyanoquinone (DDQ) oxidation
-
Build-up of double carbohelicenes using nitroarenes: dual role of the nitro functionality as an activating and leaving group作者:Fulin Zhou、Fujian Zhou、Rongchuan Su、Yudong Yang、Jingsong YouDOI:10.1039/d0sc02058c日期:——streamlined and simplified synthetic route to double carbohelicenes starting from nitroarenes through sequential nitro-activated ortho-C–H arylation, denitrative alkenylation and intramolecular cyclodehydrogenation. In this synthetic strategy, the nitro group plays a dual role namely as a leaving group for the denitrative alkenylation and as an activating group for ortho-C–H arylation, which is distinct
-
Palladium-catalyzed denitrative Sonogashira-type cross-coupling of nitrobenzenes with terminal alkynes作者:Boya Feng、Yudong Yang、Jingsong YouDOI:10.1039/c9cc08663c日期:——Described herein is a palladium-catalyzed cross-coupling reaction between nitroarenes and terminal alkynes, offering a facile method for C(sp2)-C(sp) bond formation. The utility of this protocol has been proven by the construction of polycyclic aromatic hydrocarbons (PAHs) and orthogonal cross-coupling.
-
유기발광 화합물 및 이를 포함하는 유기전계발광소자申请人:SFC CO., LTD. 에스에프씨 주식회사(120060087061) Corp. No ▼ 135511-0105889BRN ▼134-81-54429公开号:KR102271475B1公开(公告)日:2021-07-02본 발명은 유기발광 화합물에 관한 것으로서, 하기 [화학식 1]로 표시되는 화합물인 것을 특징으로 하며, 본 발명에 따른 유기발광 화합물을 채용한 유기전계발광소자는 종래 인광 발광 호스트 재료를 채용한 소자에 비하여 보다 낮은 구동 전압이 가능하여 전력효율이 우수함과 동시에 발광 효율 및 장수명을 갖는다. [화학식 1]
表征谱图
-
氢谱1HNMR
-
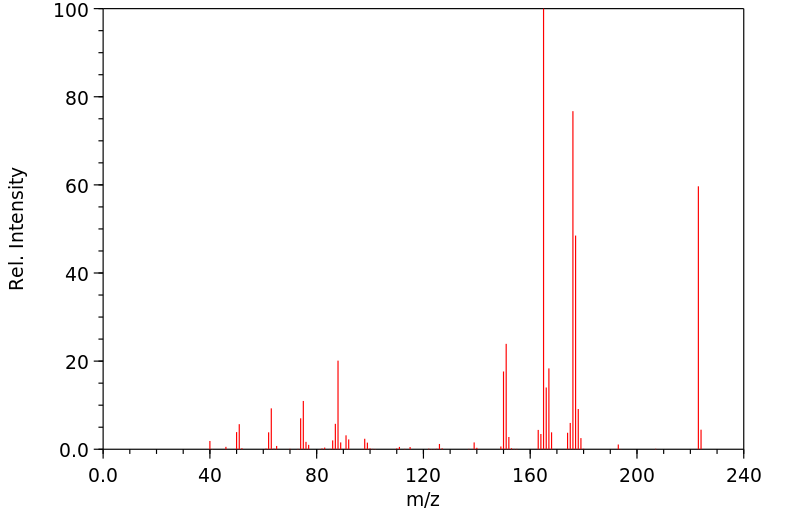
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩