DL-正肾上腺素盐酸盐 | 55-27-6
物质功能分类
中文名称
DL-正肾上腺素盐酸盐
中文别名
DL-去甲肾上腺素盐酸盐;去甲肾上腺素盐酸盐;盐酸-DL-去甲肾上腺素;DL-盐酸降肾上腺素;DL-降肾上腺素盐
英文名称
norepinephrine hydrochloride
英文别名
noradrenaline hydrochloride;dl-norepinephrine hydrochloride;(R/S)-noradrenaline hydrochloride;dl-noradrenaline hydrochloride;(+/-)-arterenol hydrochloride;(±)-1-(3,4-dihydroxyphenyl)-2-aminoethanol hydrochloride;4-(2-amino-1-hydroxyethyl)benzene-1,2-diol;hydron;chloride
CAS
55-27-6
化学式
C8H12NO3*Cl
mdl
MFCD00012880
分子量
205.641
InChiKey
FQTFHMSZCSUVEU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140-144 °C (dec.)
-
溶解度:水:可溶50mg/mL,透明至微浑浊,淡黄色至棕黄色
计算性质
-
辛醇/水分配系数(LogP):-0.68
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:86.7
-
氢给体数:5
-
氢受体数:4
安全信息
-
危险等级:6.1(b)
-
危险品运输编号:UN 2811
-
WGK Germany:3
-
海关编码:2922509090
-
危险类别:6.1(b)
-
危险类别码:R25
-
RTECS号:DN6650000
-
包装等级:III
-
储存条件:2-8°C
SDS
| Name: | DL-Norepinephrine Hydrochloride 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 55-27-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 55-27-6 | DL-Norepinephrine Hydrochloride | 99 | 200-229-8 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
The toxicological properties of this substance have not been fully investigated. Inhalation of dust may cause respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use only in a well-ventilated area.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 55-27-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 145.00 - 147.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H11NO3.HCl
Molecular Weight: 205.64
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stability unknown.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 55-27-6: DN6650000 LD50/LC50:
Not available.
Carcinogenicity:
DL-Norepinephrine Hydrochloride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: ALKALOID SALTS, SOLID, N.O.S.*
Hazard Class: 6.1
UN Number: 1544
Packing Group: III
IMO
Shipping Name: ALKALOIDS, SOLID, N.O.S.
Hazard Class: 6.1
UN Number: 1544
Packing Group: III
RID/ADR
Shipping Name: ALKALOID SALTS, SOLID, N.O.S.
Hazard Class: 6.1
UN Number: 1544
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 55-27-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 55-27-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 55-27-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:描述:DL-正肾上腺素盐酸盐 在 sodium disulfite 作用下, 以 水 为溶剂, 以55%的产率得到去甲肾上腺素杂质参考文献:名称:Synthesis and adrenoceptor affinity of some highly polar .beta.-substituted catecholamines摘要:In order to assess the potential for sympathomimetic or sympatholytic activity within the series of catecholamine beta-sulfonates 3a-c, alpha- and beta-adrenoceptor binding affinities were determined using rat brain homogenate preparations. Furthermore, their potential for indirect activity was assessed by measurement of blockade of norepinephrine uptake into rat synaptosomal preparations. Activity was uniformly low or nonexistent throughout the series. The possibility of unfavorable solution conformational distribution within the series was investigated by examination of the side chain vicinal 1H NMR coupling constants, but no differences that could account for the lack of affinity were found. The observed behavior may be due to receptor intolerance of the bulky beta-sulfonate substituent or an electronic mismatch in which normal H bonding is significantly altered.DOI:10.1021/jm00142a026
-
作为产物:描述:2-氨基-3',4'-二羟基苯乙酮盐酸盐 在 palladium 10% on activated carbon 、 氢气 作用下, 以 甲醇 为溶剂, 45.0 ℃ 、50.0 Pa 条件下, 反应 5.0h, 以96.7%的产率得到DL-正肾上腺素盐酸盐参考文献:名称:[EN] PROCESS FOR PREPARATION OF (DL) -NOREPINEPHRINE ACID ADDITION SALT, A KEY INTERMEDIATE OF (R) - (-) - NOREPINEPHRINE
[FR] PROCÉDÉ DE PRÉPARATION D'UN SEL D'ADDITION D'ACIDE DE NORÉPINÉPHRINE (DL), INTERMÉDIAIRE CLÉ DE LA NORÉPINÉPHRINE (R) - (-) -摘要:该发明揭示了一种制备(dl)-去甲肾上腺素盐的方法,通过将3,4-二羟基-α-卤代乙酰苯酮与六亚甲基四胺反应以提供六胺盐;随后进行水解和氢化。该发明还揭示了该过程中形成的一种新型中间体及其合成方法。公开号:WO2013008247A1
文献信息
-
Arylsulfonylaminobenzene derivatives and the use thereof as factor Xa申请人:3-Dimensional Pharmaceuticals, Inc.公开号:US05741819A1公开(公告)日:1998-04-21The present invention is directed to non-peptidic factor Xa inhibitors which are useful for the treatment of arterial and venous thrombotic occlusive disorders, inflammation, cancer, and neurodegenerative diseases. The factor Xa inhibitors provide compounds of structure: ##STR1## or pharmaceutically acceptable salts thereof; wherein R.sup.1 is alkyl, substituted alkyl, cycloalkyl, aryl, substituted aryl, heteroaryl or substituted heteroaryl; R.sup.2 is one of hydrogen, alkyl, cycloalkyl or aryl; R.sup.3 is one of hydrogen, hydroxy or alkoxy; R.sup.4 is one of --NH.sub.2, phenyl or pyridyl, wherein said phenyl and said pyridyl are optionally substituted with one or two of halogen, hydroxy, hydroxyalkyl, alkoxy, amino, monoalkylamino, dialkylamino, aminoalkyl, monoalkylaminoalkyl and/or dialkylaminoalkyl; X is one of --CH.sub.2 -- or --C(O)--; and n is from zero to eleven; provided that when R.sup.4 is --NH.sub.2, then R.sup.3 is hydrogen and n is other than zero; and also provided that when R.sup.3 is hydroxy or alkoxy, then R.sup.4 is other than --NH.sub.2, and n is other than zero.本发明涉及用于治疗动脉和静脉血栓性闭塞性疾病、炎症、癌症和神经退行性疾病的非肽因子Xa抑制剂。因子Xa抑制剂提供结构化合物:##STR1##或其药学上可接受的盐;其中R.sup.1是烷基、取代烷基、环烷基、芳基、取代芳基、杂环芳基或取代杂环芳基;R.sup.2是氢、烷基、环烷基或芳基中的一种;R.sup.3是氢、羟基或烷氧基中的一种;R.sup.4是--NH.sub.2、苯基或吡啶基中的一种,其中所述苯基和所述吡啶基可以选择地取代为卤素、羟基、羟基烷基、烷氧基、氨基、单烷基氨基、二烷基氨基、氨基烷基、单烷基氨基烷基和/或二烷基氨基烷基中的一种或两种;X是--CH.sub.2--或--C(O)--中的一种;n为零到十一;但是当R.sup.4为--NH.sub.2时,R.sup.3为氢且n不为零;同时当R.sup.3为羟基或烷氧基时,R.sup.4不为--NH.sub.2,且n不为零。
-
A New and Efficient Route for the Synthesis of Naturally Occurring Catecholamines作者:Roberta Bernini、Fernanda Crisante、Maurizio Barontini、Giancarlo FabriziDOI:10.1055/s-0029-1217027日期:2009.11adrenergic receptor antagonists, have been prepared by a regioselective oxidation of the corresponding 4-hydroxyphenethylamine derivatives by 2-iodoxybenzoic acid (IBX) in homogeneous as well as in heterogeneous conditions and followed by cleavage of the amino protective group. By using polymer-supported IBX, after the first oxidation, the oxidant can be recovered, regenerated, and efficiently reused for
-
Fluorometric Determination of 1,2,3,4-Tetrahydro-6,7-dihydroxyisoquinoline in Biological Materials by HPLC.作者:Akira SHIRAHATA、Masanori YOSHIOKA、Zenzo TAMURADOI:10.1248/cpb.45.1814日期:——In the belief that endogenous 1, 2, 3, 4-tetrahydro-6, 7-dihydroxyisoquinoline (DA-Fp) could be a potential marker involved in the etiology of various diseases such as Parkinsonism, we attempted to develop a fluorescence method for DA-Fp. It was synthesized by condensation of dopamine with formaldehyde according to an established method.Periodate was identified by screening from various oxidation reagents as a fluorescence reagent to DA-Fp. Optimal reaction conditions were obtained with 0.25 mM NaIO4 in 0.1 M phosphate buffer (pH 8.0) at 37°C for 15 min. The fluorescence spectrum of the derivative showed that we had found a new reaction specific for DA-Fp. This reaction we coupled on-line to high performance liquid chromatography (HPLC), which enabled us to achieve a highly sensitive method for determining DA-Fp. A working curve was linear from 2 to 800 pmol of DA-Fp per injection.To determine DA-Fp in biological materials, the pretreatment before HPLC was optimized by hydrolysis of its conjugate and suppression of the artifact with l-phenylephrine. Urinary excretion of DA-Fp in men was measured by this new present method. The urinary excretion of endogenous DA-Fp increased in a rabbit given L-DOPA. The DA-Fp concentration was determined in rat brain. The significance of DA-Fp in these biological materials is discussed and evaluated. We conclude that the present method will be useful for studying tetrahydroisoquinolines involved in meny diseases.由于认为内源性 1, 2, 3, 4-四氢-6, 7-二羟基异喹啉(DA-Fp)可能是帕金森病等多种疾病病因的潜在标志物,我们尝试开发一种 DA-Fp 的荧光方法。通过从各种氧化试剂中筛选,确定高碘酸盐为 DA-Fp 的荧光试剂。最佳反应条件是在 0.1 M 磷酸盐缓冲液(pH 8.0)中加入 0.25 mM NaIO4,在 37°C 下反应 15 分钟。衍生物的荧光光谱显示,我们发现了 DA-Fp 的新特异性反应。我们将该反应与高效液相色谱(HPLC)联用,从而实现了高灵敏度的 DA-Fp 检测方法。为了测定生物材料中的DA-Fp,我们对HPLC前的预处理进行了优化,水解了DA-Fp的共轭物,并用l-苯肾上腺素抑制了伪影。采用这种新方法测定了男性尿液中DA-Fp的排泄量。服用 L-DOPA 的兔子尿液中内源性 DA-Fp 的排泄量增加。测定了大鼠大脑中的 DA-Fp 浓度。我们讨论并评估了 DA-Fp 在这些生物材料中的意义。我们得出结论,本方法将有助于研究与多种疾病相关的四氢异喹啉类化合物。
-
[EN] COMPOSITIONS AND METHODS OF REGULATING CANCER RELATED DISORDERS AND DISEASES<br/>[FR] COMPOSITIONS ET MÉTHODES DE RÉGULATION DE TROUBLES ET MALADIES ASSOCIÉS AU CANCER申请人:ONCO THERAPIES LLC公开号:WO2017070052A1公开(公告)日:2017-04-27Provided herein are naphthylic derivative compounds, or pharmaceutically acceptable salts thereof, that are useful for inhibiting cancers. Also provided herein are methods of using effective amounts of said compounds, optionally with pharmaceutical carriers, for the treatment of cancers within human subjects.
-
Reaction of malondialdehyde with amine neurotransmitters. Formation and oxidation chemistry of fluorescent 1,4-dihydropyridine adducts作者:Marco d'Ischia、Alessandra Napolitano、Claudio CostantiniDOI:10.1016/0040-4020(95)00552-j日期:1995.8Under physiologically relevant conditions, malondialdehyde reacts smoothly with amine neurotransmitters, i.e. dopamine, norepinephrine and serotonin, to give the fluorescent dihydropyridines 1, 4 and 5, respectively, as the relatively most abundant products. Small amounts of enaminal derivatives, such as 2 and 6, could also be obtained in the reactions with dopamine and serotonin. Oxidation of 1 with
表征谱图
-
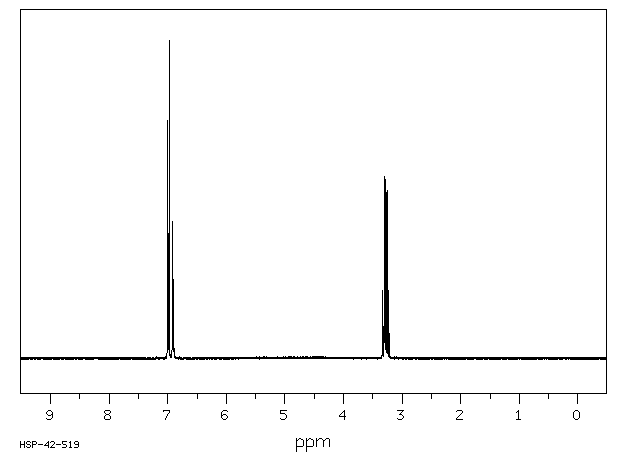
氢谱1HNMR
-
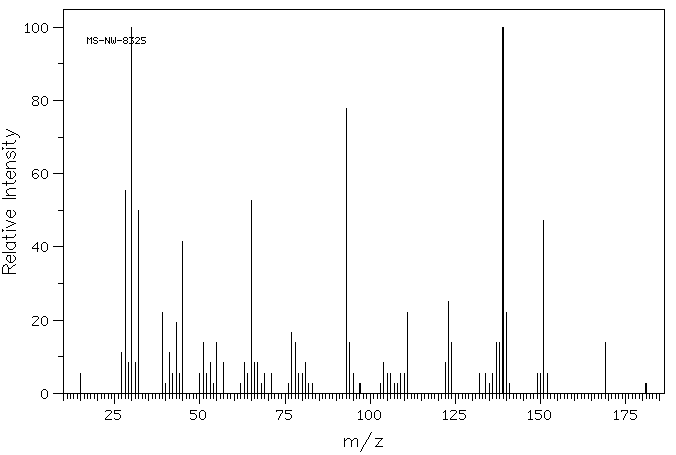
质谱MS
-
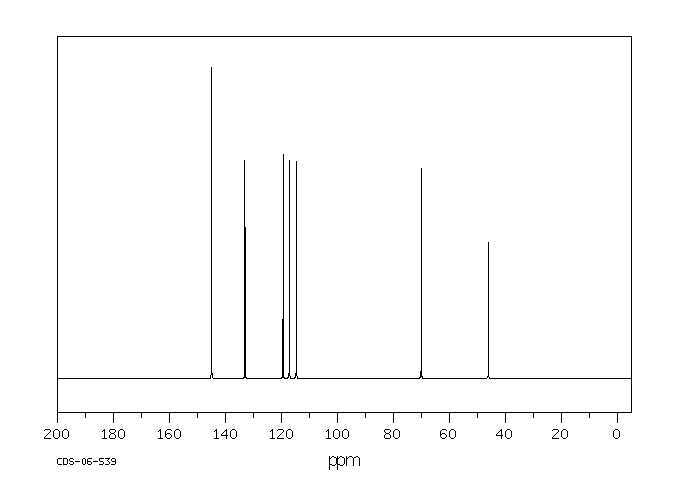
碳谱13CNMR
-
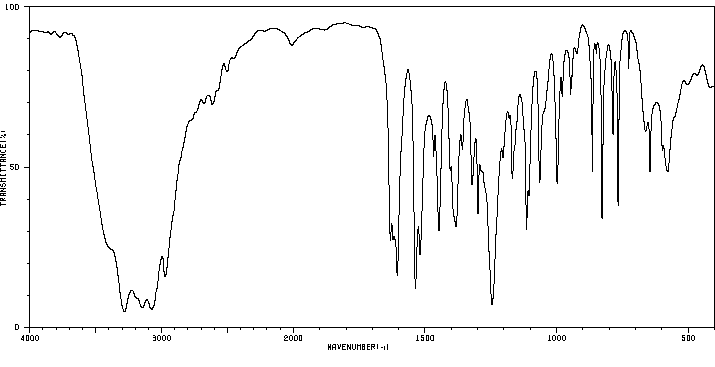
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚