DL-缬氨酸乙酯盐酸盐
中文名称
DL-缬氨酸乙酯盐酸盐
中文别名
——
英文名称
valine ethyl ester hydrochloride
英文别名
DL-valine ethyl ester hydrochloride;ethyl 2-amino-3-methylbutanoate;hydron;chloride
CAS
——
化学式
C7H15NO2*ClH
mdl
MFCD00067542
分子量
181.663
InChiKey
PQGVTLQEKCJXKF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.81
-
重原子数:11
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.857
-
拓扑面积:53.9
-
氢给体数:1
-
氢受体数:3
反应信息
-
作为反应物:描述:参考文献:名称:Organosilicon synthesis of isocyanates: IV. Synthesis of isocyanates from aliphatic and alkylaromatic amino acid esters摘要:Treatment of an alcoholic suspension of amino acids with trimethylchlorosilane yielded phenylglycine, valine, beta-phenylalanine, and homovaline ester hydrochlorides. Their saccharin-catalyzed silylation with hexamethyldisilazane proceeds quantitatively and involves only one proton of the amino group. The best conversion of the amino acid esters to the corresponding isocyanates was achieved by phosgene treatment of their monosilyl urethanes, rather than of the silylated amino esters. Monosilyl urethanes are formed quantitatively by treatment of the amino acid ester hydrochlorides with the hexamethyldisilazane-CO2 system. The H-1 NMR spectra show that monosilyl urethanes derived from alpha- and beta-amino acid esters are characterized by intramolecular interaction of the silicon atom and the oxygen atom of the carboxy group.DOI:10.1134/s1070363207040135
-
作为产物:参考文献:名称:Organosilicon synthesis of isocyanates: IV. Synthesis of isocyanates from aliphatic and alkylaromatic amino acid esters摘要:Treatment of an alcoholic suspension of amino acids with trimethylchlorosilane yielded phenylglycine, valine, beta-phenylalanine, and homovaline ester hydrochlorides. Their saccharin-catalyzed silylation with hexamethyldisilazane proceeds quantitatively and involves only one proton of the amino group. The best conversion of the amino acid esters to the corresponding isocyanates was achieved by phosgene treatment of their monosilyl urethanes, rather than of the silylated amino esters. Monosilyl urethanes are formed quantitatively by treatment of the amino acid ester hydrochlorides with the hexamethyldisilazane-CO2 system. The H-1 NMR spectra show that monosilyl urethanes derived from alpha- and beta-amino acid esters are characterized by intramolecular interaction of the silicon atom and the oxygen atom of the carboxy group.DOI:10.1134/s1070363207040135
文献信息
-
PHARMACEUTICAL COMPOUNDS AS INHIBITORS OF CELL PROLIFERATION AND THE USE THEREOF申请人:ANDERSON MARK B.公开号:US20100068197A1公开(公告)日:2010-03-18Disclosed are compounds of Formula I effective as cytotoxic agents. The compounds of this invention are useful in the treatment of a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.揭示的是作为细胞毒性剂有效的I式化合物。本发明的化合物在治疗多种临床病况中是有用的,这些病况中发生异常细胞的不受控制的生长和扩散。
-
Hypolipidemic alkenesulfonamides
-
A convenient method for the enantiomeric separation of α-amino acid esters as benzophenone imine Schiff base derivatives作者:Hu Huang、Wen Jun Xu、Jing-Yu Jin、Joon Hee Hong、Hyun-Jae Shin、Wonjae LeeDOI:10.1007/s12272-012-0609-6日期:2012.6the separation of α-amino acid esters as benzophenone Schiff base derivatives on coated chiral stationary phases (CSPs) (Chiralcel OD-H, Chiralcel OD, Chiralpak AD-H, Chiralpak AD, and Chiralpak AS) or covalently immobilized CSPs (Chiralpak IA, Chiralpak IB, and Chiralpak IC) derived from polysaccharide derivatives is described. Benzophenone imine derivatives of α-amino acid esters were readily prepared一种方便的液相色谱方法,用于在涂层手性固定相 (CSP)(Chiralcel OD-H、Chiralcel OD、Chiralpak AD-H、Chiralpak AD 和 Chiralpak AS)上或共价分离作为二苯甲酮席夫碱衍生物的 α-氨基酸酯描述了源自多糖衍生物的固定化 CSP(Chiralpak IA、Chiralpak IB 和 Chiralpak IC)。通过在 2-丙醇中搅拌二苯甲酮亚胺和 α-氨基酸酯的盐酸盐,很容易制备 α-氨基酸酯的二苯甲酮亚胺衍生物。色谱分离以1.0 mL/min的流速和254 nm的检测波长进行;0.5% 2-丙醇/己烷 (v/v) 用于 CSP。总的来说,Chiralpak IC 的分辨率优于其他 CSP。此外,
-
Efficient Synthesis of New Tetracyclic Benzofuro[3,2-<i>d</i>]-imidazo[1,2-<i>a</i>]pyrimidine-2,5-(1<i>H</i>,3<i>H</i>)-diones作者:Yanggen Hu、Min Liu、Mingwu DingDOI:10.1002/cjoc.201090072日期:2010.2with aromaic isocyanates gave carbodiimides (2), which were allowed to react further with (‐amino ester in the presence of a catalytic amount of sodium ethoxide to give selectively new tetracyclic benzofuro[3,2‐d]imidazo[1,2‐a]pyrimidine‐2,5‐(1H,3H)‐diones (5) in good yields. X‐ray structure analysis of 5i verified the proposed structure and the reaction selectivity.
-
Short Synthesis of Oxetane and Azetidine 3-Aryl-3-carboxylic Acid Derivatives by Selective Furan Oxidative Cleavage作者:Maryne A. J. Dubois、Milo A. Smith、Andrew J. P. White、Alvin Lee Wei Jie、James J. Mousseau、Chulho Choi、James A. BullDOI:10.1021/acs.orglett.0c01214日期:2020.7.17Four-membered rings remain underexplored motifs despite offering attractive physicochemical properties for medicinal chemistry. Arylacetic acids bearing oxetanes, azetidines, and cyclobutanes are prepared in two steps: a catalytic Friedel–Crafts reaction from four-membered ring alcohol substrates, followed by mild oxidative cleavage. The suitability of the products as building blocks is reflected in
表征谱图
-
氢谱1HNMR
-
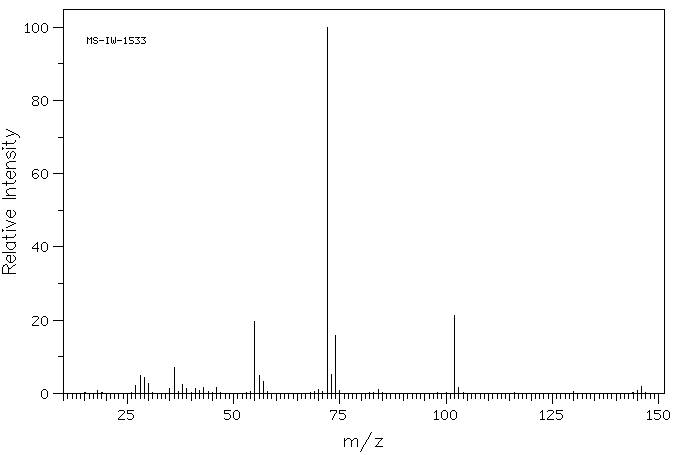
质谱MS
-
碳谱13CNMR
-
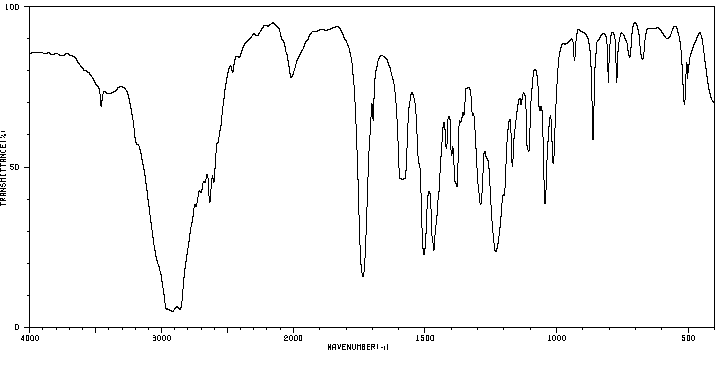
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸