N,N-二甲基乙醇胺 | 108-01-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-59 °C
-
沸点:135 °C(Press: 758 Torr)
-
密度:0.8866 g/cm3
-
物理描述:2-dimethylaminoethanol appears as a clear colorless liquid with a fishlike odor. Flash point 105°F. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion. Used to make other chemicals.
-
颜色/状态:Colorless liquid
-
气味:Amine odor
-
闪点:38 °C c.c.
-
溶解度:greater than or equal to 100 mg/mL at 73° F (NTP, 1992)
-
蒸汽密度:3.03 (NTP, 1992) (Relative to Air)
-
蒸汽压力:3.18 mm Hg at 25 °C
-
亨利常数:Henry's Law constant = 1.8X10-9 atm-cu m/mol at 25 °C (est)
-
大气OH速率常数:9.00e-11 cm3/molecule*sec
-
稳定性/保质期:
-
自燃温度:563 °F (295 °C)
-
分解:When heated to decomposition it emits toxic fumes of NOx.
-
粘度:3.5839 mPa.s at 21.6 °C
-
汽化热:42.7-43.2 kJ/mol
-
表面张力:28.2 mN/m at 20 °C
-
气味阈值:Odor Threshold Low: 0.01 [mmHg]; Odor Threshold High: 0.06 [mmHg]; Odor threshold from CHEMINFO
-
折光率:Index of refraction: 1.4300 at 20 °C
-
解离常数:9
-
保留指数:710 ;706 ;708
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:23.5
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
危险等级:8
-
安全说明:S25,S26,S36/37/39,S45
-
危险品运输编号:UN 2051 8/PG 2
-
WGK Germany:1
-
海关编码:2922192210
-
危险类别:8
-
危险品标志:C
-
危险类别码:R34,R20/21/22,R10
-
RTECS号:KK6125000
-
包装等级:II
-
储存条件:1. 储存注意事项:应储存于阴凉、通风的库房中,并远离火种和热源,库温不宜超过37℃。容器需保持密封状态,避免与氧化剂、酸类及金属粉末等物质混放。使用防爆型照明和通风设备,禁止使用易产生火花的机械设备和工具。储区应配备泄漏应急处理设备和合适的收容材料。 2. 包装采用白铁桶,每桶净重180kg。储存时需置于阴凉、通风处,并按易燃有毒化学品规定进行贮运。
SDS
| 国标编号: | 33624 |
| CAS: | 108-01-0 |
| 中文名称: | N,N-二甲基乙醇胺 |
| 英文名称: | N,N-dimethyl ethanolamine;2-dimethylamino ethyl alcohol |
| 别 名: | N,N-二甲基-2-羟基乙胺;2-二甲基氨基乙醇 |
| 分子式: | C 4 H 11 NO;(CH 3 ) 2 CNCH 2 CH 2 OH |
| 分子量: | 89.2 |
| 熔 点: | -59.0℃ 沸点:134.6? |
| 密 度: | 相对密度(水=1)0.89(2 |
| 蒸汽压: | 40℃ |
| 溶解性: | 与水混溶,可混溶于醚、芳烃 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色易挥发液体,有氨味 |
| 危险标记: | 7(易燃液体),40(有毒品) |
| 用 途: | 用作树脂原料,也用作医药、染料及油漆溶剂的原料 |
2.对环境的影响: 一、健康危害 侵入途径:吸入、食入、经皮吸收。 健康危害:本品对眼睛、皮肤、粘膜和上呼吸道有剧烈刺激作用。可致皮肤灼伤。吸入后可引起喉、支气管的炎症、水肿、痉挛,化学性肺炎、肺水肿等。对皮肤有致敏作用。 二、毒理学资料及环境行为 急性毒性:LD502340mg/kg(大鼠经口);1370mg/kg(兔经皮) 危险特性:易燃,遇高热、明火或与氧化剂接触,有引起燃烧爆炸的危险。 燃烧(分解)产物:一氧化碳、二氧化碳、氮氧化物。 3.现场应急监测方法: 4.实验室监测方法: 气相色谱法 5.环境标准: 6.应急处理处置方法: 一、泄漏应急处理 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。不要直接接触泄漏物。尽可能切断泄漏源。防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土、蛭石或其它惰性材料吸收。也可以用大量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 二、防护措施 呼吸系统防护:可能接触其蒸气时,佩戴自吸过滤式防毒面具(全面罩)。紧急事态抢救或撤离时,应该佩戴自给式呼吸器。 眼睛防护:呼吸系统防护中已作防护。 身体防护:穿胶布防毒衣。 手防护:戴橡胶手套。 其它:尽可能减少直接接触。工作现场严禁吸烟、进食和饮水。工作毕,淋浴更衣。 三、急救措施 皮肤接触:脱去被污染的衣着,用大量流动清水冲洗皮肤,至少15分钟。就医。 眼睛接触:提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。就医。 吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 食入:误服者用水漱口,给饮牛奶或蛋清。就医。 灭火方法:灭火剂:雾状水、抗溶性泡沫、干粉、二氧化碳、砂土。尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。
制备方法与用途
- 环氧乙烷法:由二甲胺与环氧乙烷进行氨化,经蒸馏、精馏、脱水而得。
- 氯乙醇法:由氯乙醇与碱进行皂化生成环氧乙烷,再与二甲胺合成得到二甲氨基乙醇。工业品二甲氨基乙醇纯度≥95%。原料消耗定额:氯乙醇(32%)5500kg/t、二甲胺(40%)2200kg/t。生产时,也可以将氯乙醇直接滴加到二甲胺中,收率为85%。精制方法为常压或减压蒸馏。
- 环氧乙烷法:由二甲胺与环氧乙烷进行氨化,经蒸馏、精馏、脱水而得。
- 氯乙醇法:由氯乙醇与碱进行皂化生成环氧乙烷,再与二甲胺合成得到二甲氨基乙醇。工业品二甲氨基乙醇纯度≥95%。原料消耗定额为氯乙醇(32%)5500kg/t、二甲胺(40%)2200kg/t。生产时,也可将氯乙醇直接滴加到二甲胺中,收率为85%。精制方法是常压或减压蒸馏。
用于合成阴离子交换树脂,水处理阳离子絮凝剂,聚氨酯催化剂,树脂助溶剂,水性体系pH调节剂,环氧树脂低温聚合促进剂,医药中间体,纺织助剂,阻蚀防垢剂及染料合成中间体等。
用途上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-甲基-2-羟基乙胺 (2-hydroxyethyl)(methyl)amine 109-83-1 C3H9NO 75.1106 N-甲基二乙醇胺 N-Methyldiethanolamine 105-59-9 C5H13NO2 119.164 三乙醇胺 triethanolamine 102-71-6 C6H15NO3 149.19 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-甲基-2-羟基乙胺 (2-hydroxyethyl)(methyl)amine 109-83-1 C3H9NO 75.1106 2-乙氧基-N,N-二甲基乙胺 N,N-dimethylaminoethylene glycol ethyl ether 26311-17-1 C6H15NO 117.191 N,N-二甲基甘氨酸 dimethylaminoacetic acid 1118-68-9 C4H9NO2 103.121 2-(二甲基氮酰)乙醇 N,N-dimethyl-mono(2-hydroxyethyl)amine-N-oxide 10489-99-3 C4H11NO2 105.137 双二甲胺基乙基醚 Bis<2-(N,N-dimethylamino)aethyl>aether 3033-62-3 C8H20N2O 160.26 —— N,N,N'-trimethylbis(aminoethyl)ether 93240-93-8 C7H18N2O 146.233 B-二甲胺基乙基 乙烯基 醚 (2-ethenyloxyethyl)dimethylamine 3622-76-2 C6H13NO 115.175
反应信息
-
作为反应物:描述:参考文献:名称:多齿醇配体稳定的氢化镓配合物:低温下Ga2O3薄膜的前体摘要:供体官能化的醇盐{我3- X N(CH 2 CH 2 O)X }(大号X ; X = 1,2,...)已被用于形成镓氢化物络合物[{GAH 2(大号1)} 2 ]和[{GaH(L 2)} 2 ]在室温下稳定且可分离。连同杂配的三(烷氧基)镓配合物[Ga(L 1)3 ]和二聚体配合物[{GaMe(L 2)} 2],这些化合物已用作通过气溶胶辅助化学气相沉积(AACVD)以甲苯为溶剂沉积Ga 2 O 3的单源前体。所得的膜大部分是透明的,表明碳污染水平低,并且它们也主要是无定形的。但是,[Ga(L 1)3 ]确实含有在450°C的基板温度下沉积的可见结晶物质,这是迄今为止对氧化镓的CVD观察到的最低值。DOI:10.1002/chem.201103380
-
作为产物:描述:参考文献:名称:Synthesis of 2-(dimethylamino)ethanol by the hydrogenolysis of tris(2-hydroxyethyl)amine摘要:本发明公开了一种在金属钯催化剂上利用分子氢将三(2-羟乙基)胺选择性氢解为 2-(二甲基氨基)乙醇的新反应。DOI:10.1039/cc9960001829
-
作为试剂:描述:碘苯 在 吡啶 、 potassium phosphate 、 copper(l) iodide 、 N,N-二甲基乙醇胺 、 草酰氯 、 4-碘甲苯 、 Olah′s reagent 、 sodium hydride 、 triethylamine tris(hydrogen fluoride) 、 N,N-二甲基甲酰胺 、 间氯过氧苯甲酸 作用下, 以 四氢呋喃 、 二氯甲烷 、 氯仿 、 水 、 mineral oil 为溶剂, 反应 72.25h, 生成参考文献:名称:Catalytic Ring Expanding Difluorination: An Enantioselective Platform to Access β,β‐Difluorinated Carbocycles摘要:
Abstract Cyclic β,β‐difluoro‐carbonyl compounds have a venerable history as drug discovery leads, but limitations in the synthesis arsenal continue to impede chemical space exploration. This challenge is particularly acute in the arena of fluorinated medium rings where installing the difluoromethylene unit subtly alters the ring conformation by expanding the internal angle (∠C−CF2−C>∠C−CH2−C): this provides a handle to modulate physicochemistry (e.g. p
K a). To reconcile this disparity, a highly modular ring expansion has been devised that leverages simple α,β‐unsaturated esters and amides, and processes them to one‐carbon homologated rings with concomitantgeminal difluorination (6 to 10 membered rings, up to 95 % yield). This process is a rare example of the formal difluorination of an internal alkene and is enabled by sequential I(III)‐enabledO ‐activation. Validation of enantioselective catalysis in the generation of unprecedented medium ring scaffolds is reported (up to 93 : 7e.r .) together with X‐ray structural analyses and product derivatization.DOI:10.1002/anie.202403957
文献信息
-
Integrase inhibitors申请人:Cai R. Zhenhong公开号:US20080058315A1公开(公告)日:2008-03-06Tricyclic compounds, protected intermediates thereof, and methods for inhibition of HIV-integrase are disclosed.三环化合物,其受保护的中间体,以及用于抑制HIV整合酶的方法被披露。
-
Triazolone derivatives申请人:Clark Richard公开号:US20080015199A1公开(公告)日:2008-01-17A Compound represented by the following general formula (1), salts thereof or hydrates of the foregoing is a novel compound useful for treatment and/or prevention of diseases associated with thrombus formation, and which is safer with suitable physicochemical stability. [wherein R 1a , R 1b , R 1c and R 1d each independently represent hydrogen, etc.; R 2 represents optionally substituted phenyl, etc.; R 3 represents optionally substituted C6-10 aryl, etc.; and Z 1 and Z 2 each independently represent hydrogen]
-
[EN] NEW COMPOUNDS I<br/>[FR] COMPOSÉS INÉDITS I申请人:BIOVITRUM AB PUBL公开号:WO2010031789A1公开(公告)日:2010-03-25The present invention relates to compounds of formula (I) and their pharmaceutically acceptable salts, solvates, hydrates, geometrical isomers, tautomers, optical isomers or N-oxides, which are inhibitors of SSAO activity. The invention further relates to pharmaceutical compositions comprising these compounds and to the use of these compounds for the treatment of medical conditions wherein inhibition of SSAO activity is beneficial, such as inflammatory diseases and immune disorders.
-
Covalent Protein Labeling by Enzymatic Phosphocholination作者:Katharina Heller、Philipp Ochtrop、Michael F. Albers、Florian B. Zauner、Aymelt Itzen、Christian HedbergDOI:10.1002/anie.201502618日期:2015.8.24present a new protein labeling method based on the covalent enzymatic phosphocholination of a specific octapeptide amino acid sequence in intact proteins. The bacterial enzyme AnkX from Legionella pneumophila has been established to transfer functional phosphocholine moieties from synthetically produced CDP‐choline derivatives to N‐termini, C‐termini, and internal loop regions in proteins of interest
-
NOVEL COMPOUNDS AS CANNABINOID RECEPTOR LIGANDS申请人:Carroll William A.公开号:US20090105306A1公开(公告)日:2009-04-23The present invention relates compounds of formula (I) wherein A and R 1 are as defined in the specification, pharmaceutical compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and pharmaceutical compositions.本发明涉及以下式(I)的化合物 其中A和R1如规范中所定义,包括这些化合物的药物组合物,以及使用这些化合物和药物组合物治疗疾病和疾病的方法。
表征谱图
-
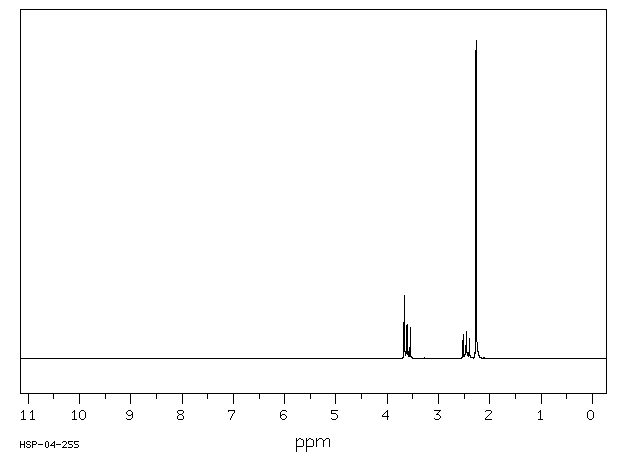
氢谱1HNMR
-
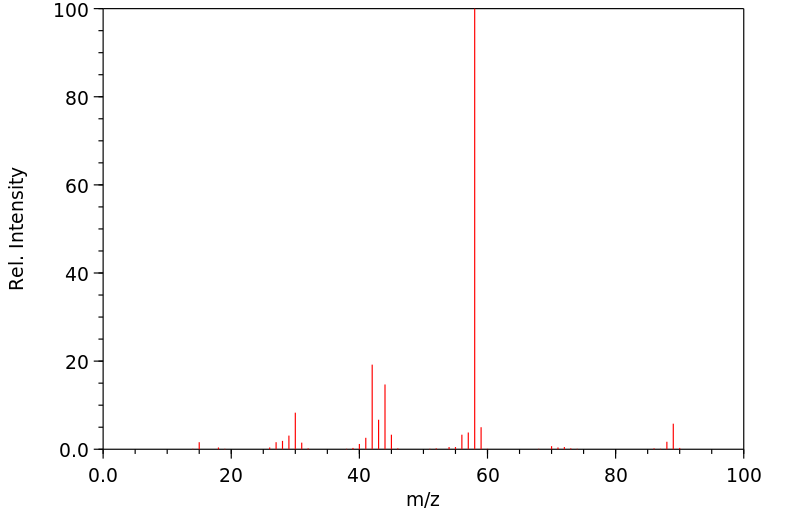
质谱MS
-
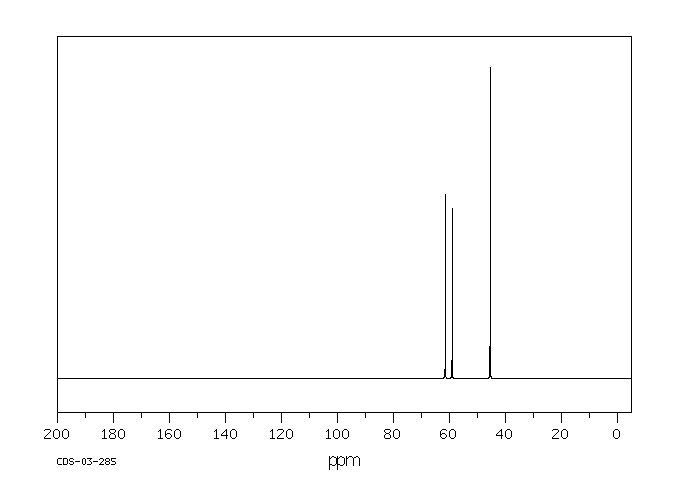
碳谱13CNMR
-
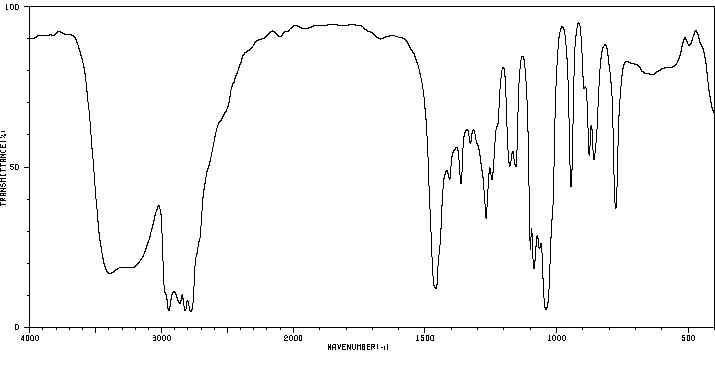
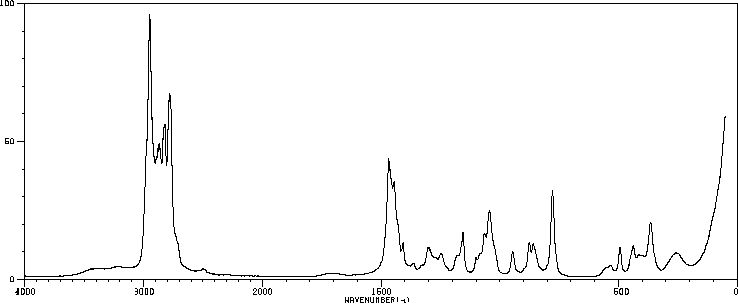
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息