敌瘟磷 | 17109-49-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:25°C
-
沸点:bp0.01 154°
-
密度:d420 1.23
-
物理描述:Solid
-
颜色/状态:Yellow to light brown liquid... .
-
气味:Characteristic odor
-
溶解度:In n-hexane 20-50, dichloromethane, isopropanol, toluene 200 (all in g/l, 20 °C). Readily soluble in methanol, acetone, benzene, xylene, carbon tetrachloride and dioxane. Sparingly soluble in heptane.
-
蒸汽压力:2.70X10-7 mm Hg @ 25 °C
-
稳定性/保质期:
Stable in neutral media. Hydrolysed by strong acids and alkalis; at 25 °C, DT50 19 days (pH 7), 2 days (pH 9). Degradable in the presence of light.
-
分解:At 25 °C decomposition occurs in 49 hr at pH 9 and in 1,135 hr at pH 7.
-
保留指数:2295.7;2277.8;2329.6;2359
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:19
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:76.9
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:N,T
-
安全说明:S36/37,S45,S60,S61
-
危险类别码:R21,R50/53,R43,R23/25
-
WGK Germany:3
-
危险品运输编号:UN 2810
-
RTECS号:TE3850000
-
海关编码:2930909058
-
包装等级:III
-
危险类别:6.1(b)
制备方法与用途
毒性
纯品对大鼠急性经口LD50为312 mg/kg,小鼠为214 mg/kg;大鼠急性经皮LD50为1230 mg/kg(4小时),小鼠皮下注射LD50为163 mg/kg。对皮肤和眼睛无致突变作用,三代繁殖试验和神经毒性试验均未见异常。
化学性质
工业品呈黄色至浅棕色透明液体,并带有恶臭味。沸点154℃/1.33Pa,相对密度1.23(20℃),折射率nD22为1.61。能溶于甲醇、乙醚、氯仿、丙酮、苯、二甲苯等有机溶剂,难溶于水。在25℃时半衰期分别为:pH=7时为1.135小时,pH=9时为49小时。酸性条件下较稳定,在碱性条件尤其是高温下易发生水解或酯交换反应。
用途
敌瘟磷是一种广谱有机磷杀菌剂,具有内吸作用,兼有保护和治疗功能。主要用于防治稻瘟病,对水稻叶瘟、穗颈瘟、苗瘟均具有良好防效。预防叶瘟时,使用40%乳油7.5~10.5 mL/100 m2喷雾;重发情况下则需增加剂量至11.3~15 mL/100 m2,每隔10~14天施药一次。同样剂量可用于防治穗颈瘟;种子处理可预防苗瘟。此外还可用于防治麦类赤霉病、水稻小粒菌核病、水稻纹枯病、玉米大斑病和小斑病、胡麻叶斑病以及稻叶蝉、飞虱、稻螟等害虫。本药剂在病原菌侵入前施用效果更佳。
用途
该品是一种有机磷杀菌剂,主要用于防治稻瘟病,并兼治稻胡麻叶斑病、稻小粒菌核病、纹枯病等疾病,同时也具有防除稻飞虱和叶蝉的作用。大鼠口服LD50为214 mg/kg。
用途
主要用以防治稻瘟病,可兼治稻胡麻叶斑病、稻小粒菌核病、纹枯病等疾病,并具有防治稻飞虱和叶蝉的效果。
生产方法
由硫酚钠盐与二氯磷酸乙酯反应制得敌瘟磷。具体步骤如下:
- O-乙基磷酰二氯的制备:三氯氧磷与无水乙醇作用,无水乙醇稍过量,生成O-乙基磷酰二氯。
- 苯硫酚钠的制备:苯氯磺化得到苯磺酰氯,在硫酸和锌粉(或铁粉)存在下转化为苯硫酚,并中和得相应的酚钠。
- 敌瘟磷合成:将O-乙基磷酰二氯与苯硫酚钠作用,最终生成敌瘟磷。
类别
农药
毒性分级
高毒
急性毒性
口服-大鼠 LD50: 100 毫克/公斤;口服-小鼠 LD50: 143 毫克/公斤
可燃性危险特性
明火可燃,受热分解产生有毒的氧化磷、氧化硫和氧化氮气体。
储运特性
库房应通风低温干燥,并与食品原料分开存放运输。
灭火剂
砂土、干粉、泡沫
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— thiophosphoric acid O-ethyl ester-S-phenyl ester 38902-34-0 C8H11O3PS 218.213
反应信息
-
作为反应物:描述:参考文献:名称:MURAI, TOSHINOBU, BULL. NAT. INST. AGR. SCI., 1983, N 37, 91-125摘要:DOI:
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
表征谱图
-
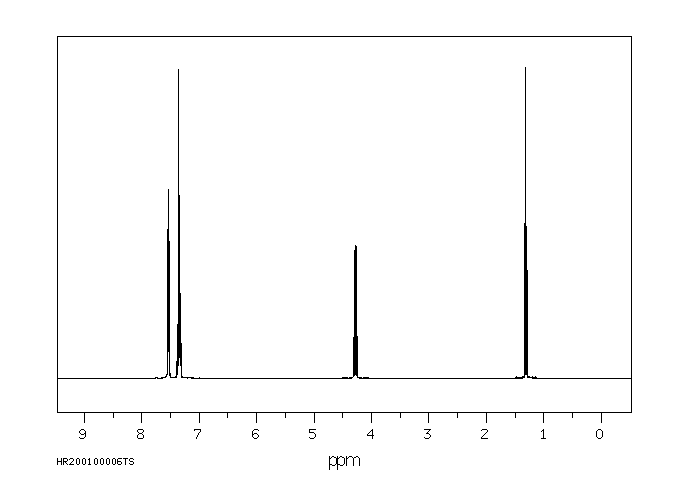
氢谱1HNMR
-
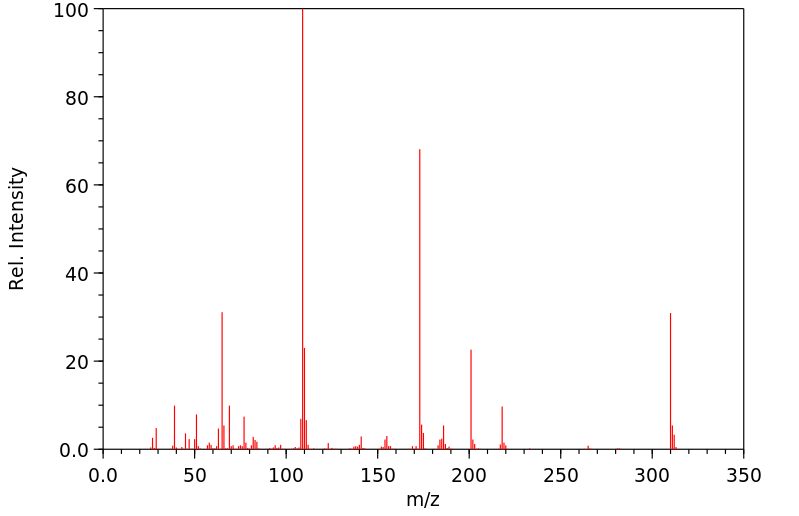
质谱MS
-
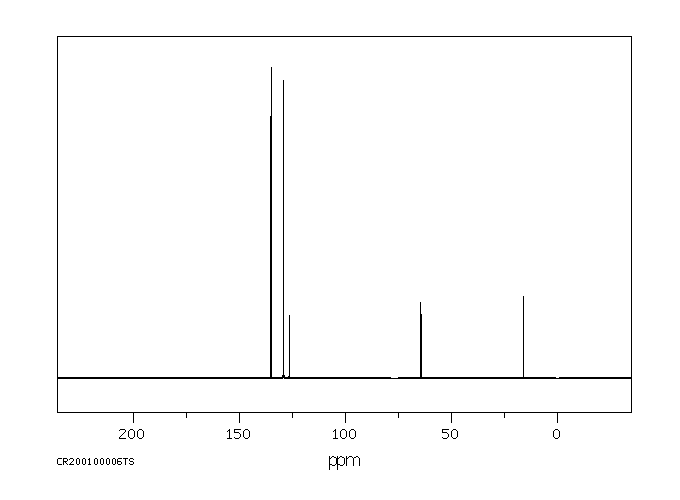
碳谱13CNMR
-
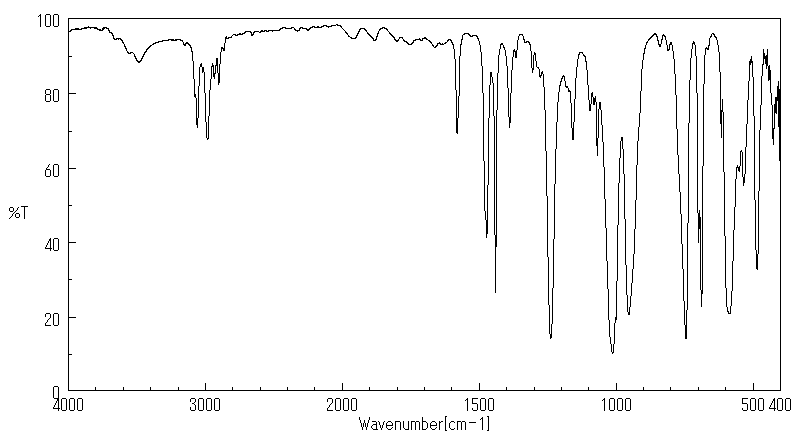
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息