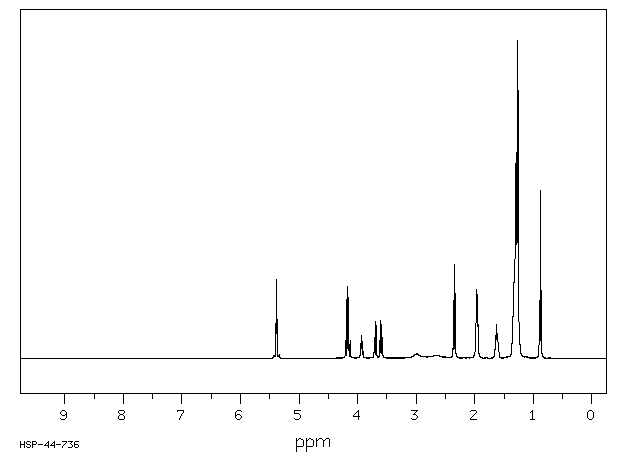
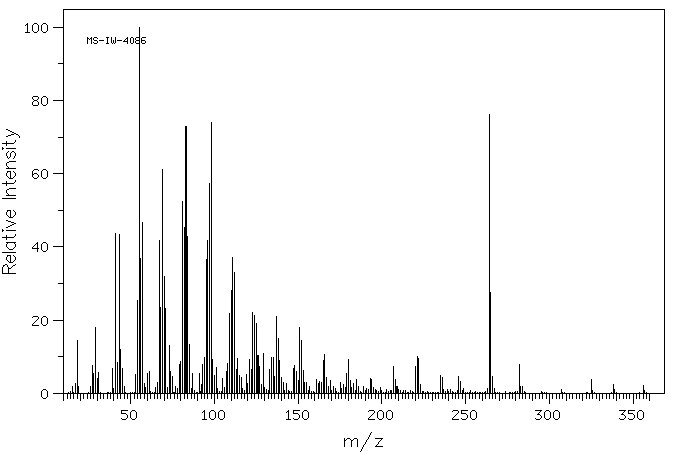
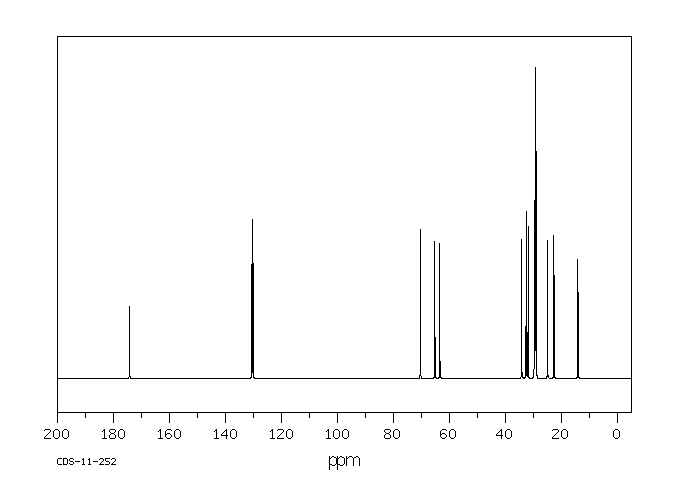
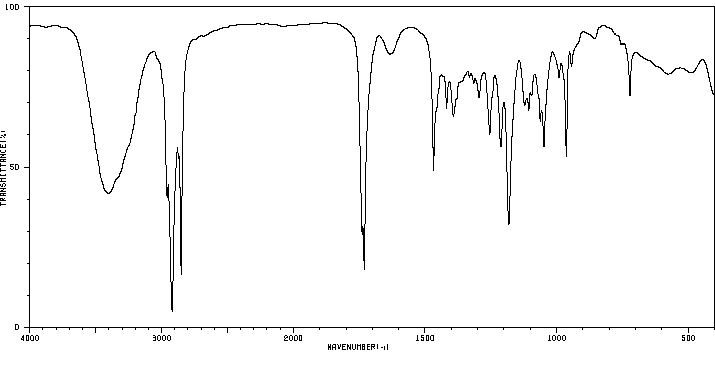
毒理性
TREATMENT OF HUMAN ERYTHROCYTE GHOST MEMBRANES WITH THE FUSOGENIC LIPID GLYCEROL MONOOLEATE INCREASED THE FLUIDITY OF THE MEMBRANE LIPIDS, BUT THE NONFUSOGENIC LIPID GLYCEROL MONOSTEARATE HAD NO EFFECT.
来源:Hazardous Substances Data Bank (HSDB)