2-二甲基氨基乙酰肼盐酸盐 | 539-64-0
中文名称
2-二甲基氨基乙酰肼盐酸盐
中文别名
GIRARD'S试剂;吉拉尔特试剂
英文名称
N,N-dimethylglycine hydrazide hydrochloride
英文别名
Girard-D-Reagenz;N,N-dimethylaminoacetohydrazide hydrochloride;dimethylaminoacetic hydrazide hydrochloride;N,N-Dimethyl-glycin-hydrazid; Hydrochlorid;N,N-dimethylglycyl hydrazine hydrochloride;dimethylaminoacethydrazide hydrochloride;dimethylaminoacethydrazide HCl;2-(dimethylamino)acetohydrazide;hydron;chloride
CAS
539-64-0
化学式
C4H11N3O*ClH
mdl
MFCD00042020
分子量
153.612
InChiKey
OBXBQDQBTXIDCA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:208 °C
计算性质
-
辛醇/水分配系数(LogP):-1.44
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:58.4
-
氢给体数:3
-
氢受体数:3
安全信息
-
危险品标志:Xn,F
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2928000090
SDS
| Name: | GIRARD REAGENT D 98% (TITR.) Material Safety Data Sheet |
| Synonym: | |
| CAS: | 539-64-0 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 539-64-0 | GIRARD REAGENT D, 98% (TITR.) | 98 | 208-721-4 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 539-64-0: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 185 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C4H11N3O.HCl
Molecular Weight: 117.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 539-64-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
GIRARD REAGENT D, 98% (TITR.) - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 539-64-0: No information available.
Canada
CAS# 539-64-0 is listed on Canada's NDSL List.
CAS# 539-64-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 539-64-0 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:N-叔丁氧羰基-L-丙氨酸 、 2-二甲基氨基乙酰肼盐酸盐 在 1-羟基苯并三唑 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 N,N-二异丙基乙胺 作用下, 以 二氯甲烷 为溶剂, 反应 24.0h, 以57%的产率得到{(S)-2-[N’-(2-dimethylaminoacetyl)hydrazino]-1-methyl-2-oxoethyl}carbamic acid tert-butyl ester参考文献:名称:NOVEL COMPOUNDS摘要:本文定义的式(I)的吡唑酮衍生物具有人类中性粒细胞弹性蛋白酶抑制性质,并可用于治疗与HNE有关的疾病或症状。公开号:US20170340611A1
-
作为试剂:描述:3,5-双三氟甲基苄基溴 在 4-二甲氨基吡啶 、 18-冠醚-6 、 phosphorus pentoxide 、 2-二甲基氨基乙酰肼盐酸盐 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 三乙胺 、 三氟乙酸 作用下, 以 四氢呋喃 、 二氯甲烷 、 xylene 为溶剂, 反应 9.08h, 生成 (1R*,2R*,5S*,6R*)-8-benzyl-2-{[3,5-Bis(trifluoromethyl)phenyl]methoxy}-6-[(5-dimethylaminomethyl)oxa-3,4-diazo-2-yl]-1-phenyl-8-azabicyclo[3,2.1] octane参考文献:名称:[EN] 8-AZABICYCLO[3.2.1]OCTANE ETHER DERIVATIVES AND THEIR USE AS NEUROKININ RECEPTOR ANTAGONISTS
[FR] DERIVES D'ETHER 8-AZABICYCLO[3.2.1]OCTANE ET LEUR UTILISATION EN TANT QU'ANTAGONISTES DE RECEPTEUR DE NEUROKININE摘要:本发明涉及以下式(I)的化合物:其中X代表氢,C1-4烷基,可选择地被羟基,甲氧基或苄氧基取代,或CO2(C1-2烷基);Z为-CR9R10CH2-或-CH2CR9R10-;而R1、R2、R3、R4、R5和R6如本文所定义。这些化合物在治疗或预防抑郁症、焦虑、疼痛、炎症、偏头痛、呕吐或带状疱疹后神经痛方面具有特殊用途。公开号:WO2004031185A1
文献信息
-
N-Dimethylglycin-hydrazid-hydrochlorid, Reagenz zur Isolierung und Charakterisierung von Carbonyl-Derivaten
-
N-(Acetamido)thiourea based simple neutral hydrogen-bonding receptors for anions作者:Wen-Xia Liu、Rui Yang、Ai-Fang Li、Zhao Li、Yu-Feng Gao、Xing-Xing Luo、Yi-Bin Ruan、Yun-Bao JiangDOI:10.1039/b910255h日期:——of a proline derivative 6 bearing a chiral center in the N-amido moiety provide direct evidence for this conformation change upon its binding with anions in MeCN. The amplified effect of substituent X at the N′-phenyl ring of 5 on the anion binding constant supports the conclusion of anion-binding switched charge transfer in the anion binding complex. 1H NMR and absorption titrations for 5 indicatedN-(乙酰氨基)-N'-苯硫脲(4–6)被发现是有效的阴离子受体,其阴离子亲和力比它们的阴离子亲和力高N-苯甲酰胺基-N'-苯硫脲对应对象(1和2)。这N'-苯硫脲在4–6中的部分被证明是发色团,其最大吸收在。270纳米 已经发现,在阴离子的存在下,在约1μm的吸收。270 nm的4–6(5f除外)in乙腈(MeCN)呈蓝色偏移并增强,而大约在2020年出现红移的肩膀。295 nm,以及大约等渗点。240纳米 1:1的阴离子结合的常数4-6在10,例如6 -10 7中号-1数量级为ACO -在MeCN,被认为是比那些更高1和2,虽然硫脲基的酸度4–6中的-NH质子低于1和2中的-NH质子。1 H NMR数据表明4–6中的N–N单键是扭曲的,但小于1中的N–N单键。和2。建议在阴离子结合后在4–6的N–N单键处发生构象变化,这导致阴离子结合复合物中的平面氢键网络发生电荷转移,其中N-酰基部分
-
Generation of dynamic combinatorial libraries and assessment thereof by deconvolution申请人:Therascope AG公开号:EP1184359A1公开(公告)日:2002-03-06The present invention deals with a method for the generation of a dynamic combinatorial library of ligands for a given target, capable of binding at least two functionalities, which method comprises the following steps: (v)selecting a plurality of functionalities which may bind to the target; (vi)selecting a set of n molecules carrying said functionalities, which molecules are capable of reversibly associating with each other under formation of an entity, n being an integer ≥ 1; (vii)mixing together said set of said n molecules; (viii)subjecting the mixture to conditions allowing a reversible bond formation and cleavage under formation of entities carrying different combinations of functionalities, until equilibrium is reached; (v)optionally transforming the said entities into ligands; (vi)repeating steps (ii) to (v) n-times, each time a different molecule of said set of n molecules being omitted; (vii)assessing the ability of each of the mixtures obtained to bind to a given target.
-
Benzo(b)pyrrolobenzodiazepines申请人:Hoechst-Roussel Pharmaceutical Inc.公开号:US04983601A1公开(公告)日:1991-01-08Novel benzo[b]pyrrolo[3,2,1-jk]benzodiazepines, intermediates and processes for the preparation thereof, and methods for alleviating pain utilizing the compounds or compositions thereof are disclosed.
-
Harnessing chemoselective imine ligation for tethering bioactive molecules to platinum(iv) prodrugs作者:Daniel Yuan Qiang Wong、Jia Yi Lau、Wee Han AngDOI:10.1039/c2dt30264k日期:——bioactive molecules to the axial positions of platinum(IV) prodrugs suffer from limited scope, poor yields and low reliability. This report explores the applications of current chemoselective ligation chemistries to platinum(IV) anticancer complexes with the aim of addressing the aforementioned limitations. Here, we describe the synthesis of a platinum(IV) complex bearing an aromatic aldehyde functionality铂金(II)抗癌药是最有效且最常用的化学治疗药物之一。近年来,人们越来越关注开发惰性铂(IV)支架作为减轻铂(II)抗癌复合物局限性的前药策略。在这种前药策略中,轴向配体在细胞内还原为活性铂(II)同类物时随之释放,从而提供了缀合生物活性辅药的可能性,这些药物可以协同增强癌细胞的细胞毒性。将生物活性分子束缚到铂的轴向位置的现有技术(IV)前药的作用范围有限,产量低且可靠性低。本报告探讨了目前的化学选择性连接化学在铂(IV)抗癌复合物中的应用,旨在解决上述局限性。在这里,我们描述了带有芳香醛官能团的铂(IV)配合物的合成,并探讨了亚胺与各种酰肼和氨氧基官能化底物的连接范围。作为概念的证明,我们将抗炎蛋白ANXA1的六序列长肽模拟物(AMVSEF)拴在一起。
表征谱图
-
氢谱1HNMR
-
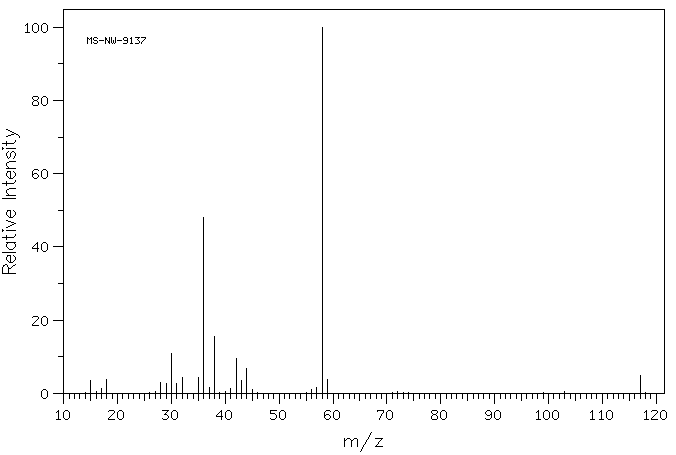
质谱MS
-
碳谱13CNMR
-
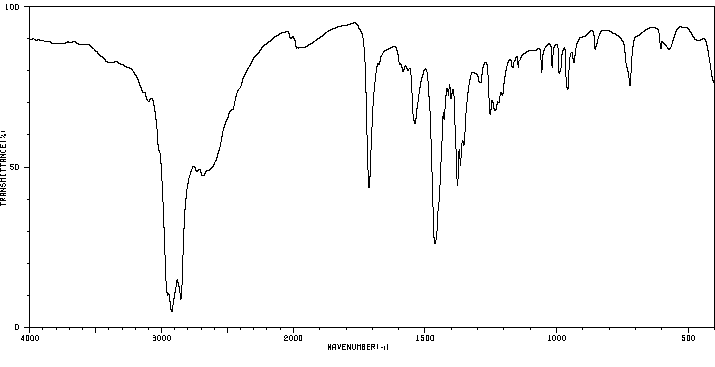
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸