2-甲酰胺基-2-苯基丙酸 | 3381-60-0
中文名称
2-甲酰胺基-2-苯基丙酸
中文别名
——
英文名称
2-formylamino-2-phenyl-propionic acid
英文别名
2-Formylamino-2-phenyl-propionsaeure;2-Formamino-2-phenyl-propionsaeure;(+/-)-2-Formylamino-2-phenyl-propionsaeure;α-Formamino-α-phenyl-propionsaeure;N-Formyl-2-phenylalanin;N-Formyl-2-phenylalanine;2-formamido-2-phenylpropanoic acid
CAS
3381-60-0
化学式
C10H11NO3
mdl
——
分子量
193.202
InChiKey
QLRKSVGTYRLLEP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:66.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2924299090
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 DL-2-氨基-2-苯基丙酸 2-amino-2-phenylpropanoic acid 565-07-1 C9H11NO2 165.192 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (S)-2-formylamino-2-phenyl-propionic acid 33228-58-9 C10H11NO3 193.202 —— methyl rac-2-benzyl-2-formamidopropionat 2683-73-0 C11H13NO3 207.229
反应信息
-
作为反应物:参考文献:名称:Process for obtaining a 5-phenyl-5-lower alkyl hydantoin摘要:公开号:US02561284A1
-
作为产物:描述:参考文献:名称:McKenzie; Clough, Journal of the Chemical Society, 1912, vol. 101, p. 393摘要:DOI:
文献信息
-
Über die Stereochemie der Hydrogenolyse von N-Benzyl-Bindungen I. Die Hydrogenolyse von Derivaten der 2-Amino-2-phenyl-propionsäure.作者:H. Dahn、J. A. Garbarino、C. O'MurchuDOI:10.1002/hlca.19700530616日期:——The stereochemistry of the hydrogenolysis of benzyl-N bonds was studied using S(+)-2-dimethylamino-2-phenyl-propionic acid (I) and its derivatives, and R(−)-2-anilino-2-phenyl-propionic acid (II). The configuration of I was confirmed, that of II established by ORD. measurements, after transformation of the phenyl into cyclohexyl groups. On a palladium catalyst the hydrogenolysis of I, its methyl and
-
ANTI-VIRAL COMPOUNDS申请人:Betebenner A. David公开号:US20070232627A1公开(公告)日:2007-10-04Compounds effective in inhibiting replication of Hepatitis C virus (“HCV”) or other viruses are disclosed. This invention is also directed to compositions comprising such compounds, co-formulation or co-administration of such compounds with other anti-viral or therapeutic agents, processes and intermediates for the syntheses of such compounds, and methods of using such compounds for the treatment of HCV or other viral infections.本发明公开了一种有效抑制丙型肝炎病毒(“HCV”)或其他病毒复制的化合物。本发明还涉及包含这些化合物的组合物、这些化合物与其他抗病毒或治疗剂的共同配方或共同管理、用于合成这些化合物的过程和中间体,以及使用这些化合物治疗HCV或其他病毒感染的方法。
-
Anti-viral compounds申请人:Abbott Laboratories公开号:US07910595B2公开(公告)日:2011-03-22Compounds effective in inhibiting replication of Hepatitis C virus (“HCV”) or other viruses are disclosed. This invention is also directed to compositions comprising such compounds, co-formulation or co-administration of such compounds with other anti-viral or therapeutic agents, processes and intermediates for the syntheses of such compounds, and methods of using such compounds for the treatment of HCV or other viral infections.本发明揭示了一些有效抑制丙型肝炎病毒(“HCV”)或其他病毒复制的化合物。本发明还涉及包含这些化合物的组合物,这些化合物与其他抗病毒或治疗剂一起配方或联合使用,用于合成这些化合物的过程和中间体,以及使用这些化合物治疗HCV或其他病毒感染的方法。
-
EKSTROEM; GOMEZ REVILLA; MOLLBERG, Acta Chemica Scandinavica (1947), 1965, vol. 19, p. 281 - 299作者:EKSTROEM、GOMEZ REVILLA、MOLLBERG、THELIN、SJOEBERGDOI:——日期:——
-
LOW AFFINITY SCREENING METHOD申请人:Graffinity Pharmaceuticals Aktiengesellschaft公开号:EP1360489A1公开(公告)日:2003-11-12
表征谱图
-
氢谱1HNMR
-
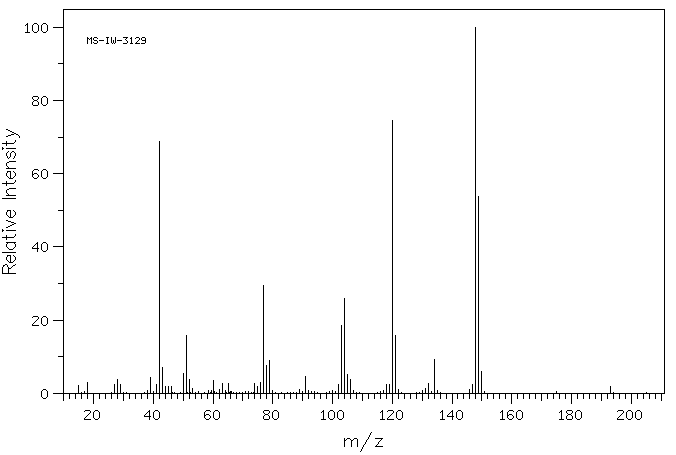
质谱MS
-
碳谱13CNMR
-
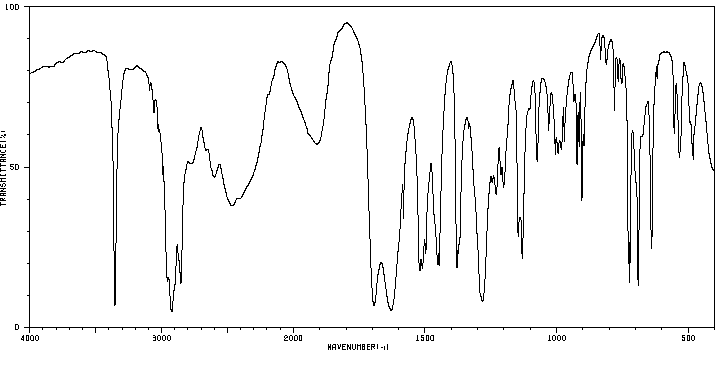
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸