2-butyl-6-methylpyrimidin-4(3H)-one | 90565-51-8
中文名称
——
中文别名
——
英文名称
2-butyl-6-methylpyrimidin-4(3H)-one
英文别名
2-butyl-4-methyl-1,6-dihydro-6-pyrimidone;2-n-butyl-6-methylpyrimidin-4(3H)-one;2-butyl-6-methyl-3H-pyrimidin-4-one;2-Butyl-6-methyl-3H-pyrimidin-4-on;2-butyl-6-methyl-1H-pyrimidin-4-one
CAS
90565-51-8
化学式
C9H14N2O
mdl
——
分子量
166.223
InChiKey
HEFURGIWSUMDFP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:120 °C
-
沸点:256.0±23.0 °C(Predicted)
-
密度:1.08±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.56
-
拓扑面积:41.5
-
氢给体数:1
-
氢受体数:2
SDS
反应信息
-
作为反应物:描述:2-butyl-6-methylpyrimidin-4(3H)-one 在 甲醇 、 Lindlar's catalyst 、 三氯氧磷 作用下, 生成 2-butyl-4-methyl-pyrimidine参考文献:名称:Yanai; Naito, Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1941, vol. 61, p. 99,105; dtsch. Ref. S. 46, 52摘要:DOI:
-
作为产物:描述:N-(3-oxobutanoyl)pentanamide 在 ammonium acetate 作用下, 以 溶剂黄146 为溶剂, 反应 0.25h, 以92%的产率得到2-butyl-6-methylpyrimidin-4(3H)-one参考文献:名称:A facile synthesis of pyrimidin-4-ones摘要:A facile synthesis of 2,6-disubstituted pyrimidin-4-ones and 2,5,6-trisubstituted pyrimidin-4-ones from commercially available materials with application of microwave technology in key steps is described. (C) 2009 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2009.11.096
文献信息
-
N-3-Substituted Pyrimidinones as Potent, Orally Active, AT1 Selective Angiotensin II Receptor Antagonists作者:Aldo Salimbeni、Renato Canevotti、Fabio Paleari、Davide Poma、Saturnino Caliari、Francesco Fici、Rocco Cirillo、Anna R. Renzetti、Alessandro SubissiDOI:10.1021/jm00024a008日期:1995.11A novel series of nonpeptide angiotensin II (A II) antagonists containing a pyrimidinone ring which carries a C-linked biphenyltetrazole moiety and a carboxyheteroaryl group on the 3-position have been prepared. Their affinity for the AT1 receptor was determined in a binding assay on rat adrenal cortical membranes. The in vivo antihypertensive properties were tested by evaluating the inhibition of制备了一系列新的非肽血管紧张素II(A II)拮抗剂,该拮抗剂包含嘧啶酮环,该嘧啶酮环在3位上带有C连接的联苯四唑部分和羧基杂芳基。通过对大鼠肾上腺皮质膜的结合试验确定它们对AT1受体的亲和力。通过评估对A II的升压反应的抑制,然后进行静脉和内服给药,来测试体内的抗高血压特性。与其他已知的A II拮抗剂(例如DUP)相比,广泛的分子建模研究(包括分子静电势分布的比较,构象分析和A II计算药效团模型上的覆盖物)用于评估新化合物的结构参数。 -753和SK&F 108566)。根据建模研究,在嘧啶酮环的3-位引入(羧基杂芳基)甲基部分导致衍生物的效力增加。甲基2-[[[4-丁基-2-甲基-6-氧代-5-] [[2'-(1H-四唑-5-基)] [1,1'-联苯] -4-基]甲基] -1 -(6H)-嘧啶基]甲基] -3-噻吩羧酸酯(3k,LR-B / 081),是该系列中最有效的化合物之一(Ki
-
Substituted pyrimidinones as angiotensin II antagonists
-
Synthesis and biological activity of novel pyrimidinone containing thiazolidinedione derivatives作者:Gurram R Madhavan、Ranjan Chakrabarti、Reeba K Vikramadithyan、Rao N.V.S Mamidi、V Balraju、B.M Rajesh、Parimal Misra、Sunil K.B Kumar、Braj B Lohray、Vidya B Lohray、Ramanujam RajagopalanDOI:10.1016/s0968-0896(02)00107-4日期:2002.8A series of pyrimidinone derivatives of thiazolidinediones were synthesized. Their biological activity were evaluated in insulin resistant, hyperglycemic and obese db/db mice. In vitro PPARgamma transactivation assay was performed in HEK 293T cells. PMT13 showed the best biological activity in this series. PMT13 (5-[4-[2-[2-ethyl-4-methyl-6-oxo-1,6-dihydro-1-pyrimidinyl]ethoxy]phenylmethyl]thiazolidine-2合成了一系列噻唑烷二酮的嘧啶酮衍生物。在胰岛素抵抗,高血糖和肥胖的db / db小鼠中评估了它们的生物学活性。在HEK 293T细胞中进行了体外PPARγ激活测定。PMT13在该系列中表现出最好的生物活性。PMT13(5- [4- [2- [2-乙基-4-甲基-6-氧代-1,6-二氢-1-嘧啶基]乙氧基]苯基甲基]噻唑烷-2,4-二酮)血浆葡萄糖水平更高, db / db小鼠的甘油三酸酯和胰岛素降低活性高于罗格列酮和吡格列酮。PMT13显示出比标准化合物更好的PPARγ反式激活。Wistar大鼠的药代动力学研究表明,PMT13具有良好的全身性暴露。在Wistar大鼠中进行的28天口服毒性研究未显示任何与治疗相关的不良反应。
-
Heterocyclic compounds, process for their preparation and pharmaceutical compositions containing them and their use in the treatment of diabetes and related diseases申请人:Dr. Reddy's Research Foundation公开号:US06310069B1公开(公告)日:2001-10-30The present invention relates to novel antidiabetic compounds, their tautomeric forms, their derivatives, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, their pharmaceutically acceptable solvates and pharmaceutically acceptable compositions containing them. This invention particularly relates to novel azolidinedione derivatives of the general formula (I), and their pharmaceutically acceptable salts, pharmaceutically acceptable solvates and pharmaceutical compositions containing them.
-
Heterocyclic compounds, process for their preparation and pharmaceutical申请人:Dr. Reddy's Research Foundation公开号:US05885997A1公开(公告)日:1999-03-23The present invention relates to novel antidiabetic compounds, their tautomeric forms, their derivatives, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, their pharmaceutically acceptable solvates and pharmaceutically acceptable compositions containing them. This invention particularly relates to novel azolidinedione derivatives of the general formula (I), and their pharmaceutically acceptable salts, pharmaceutically acceptable solvates and pharmaceutical compositions containing them. ##STR1##
表征谱图
-
氢谱1HNMR
-
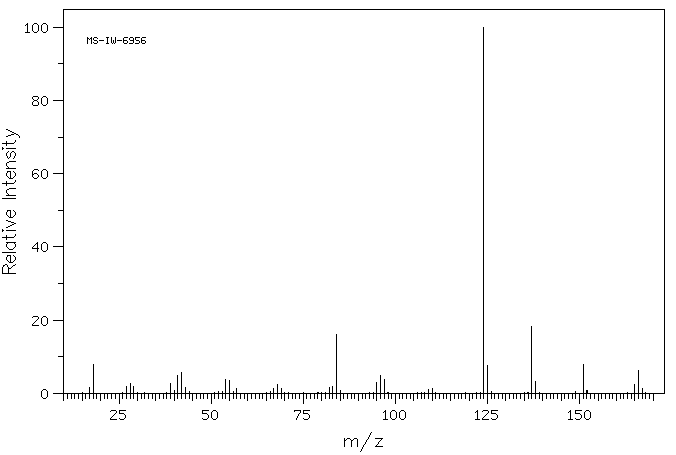
质谱MS
-
碳谱13CNMR
-
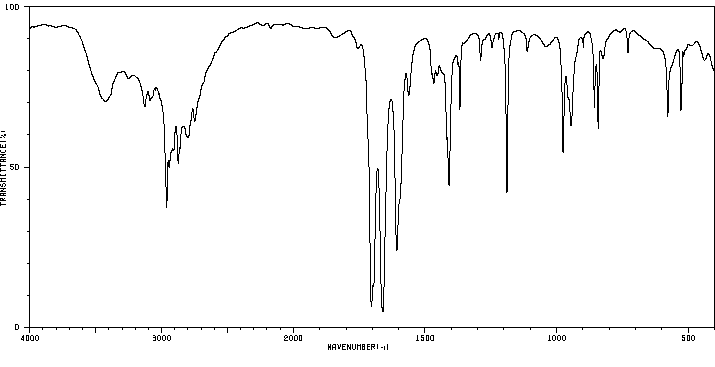
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3