1,4-dichlorophenazine | 2881-86-9
中文名称
——
中文别名
——
英文名称
1,4-dichlorophenazine
英文别名
2,5-dichlorophenazine;1,4-dichloro-phenazine;1,4-Dichlor-phenazin;1,4-Dichlorphenazin
CAS
2881-86-9
化学式
C12H6Cl2N2
mdl
——
分子量
249.099
InChiKey
JBXONTRNJUKZTB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,4,6,9-Tetrachlor-phenazin 3129-40-6 C12H4Cl4N2 317.989
反应信息
-
作为反应物:描述:参考文献:名称:Maffei et al., Gazzetta Chimica Italiana, 1953, vol. 83, p. 812,816摘要:DOI:
-
作为产物:描述:3,3,6,6-四氯-1,2-环己二酮 在 吡啶 、 sodium acetate 、 lithium perchlorate 作用下, 以 溶剂黄146 、 N,N-二甲基甲酰胺 、 乙腈 、 苯 为溶剂, 反应 24.0h, 生成 1,4-dichlorophenazine参考文献:名称:A new synthetic route to 1-chlorophenazines. The electrochemical monodechlorination of 3,3,6,6-tetrachloro-1,2-cyclohexanedione as a key step摘要:A convenient new method for the synthesis of 1-chlorophenazines has been established. The first step involves an almost quantitative electrochemical reduction of 3,3,6,6-tetrachloro-1,2-cyclohexanedione 1 to 3,6,6-trichloro-2-hydroxy-2-cyclohexen-1-one 2. The reaction of 2 with aromatic 1,2-diamines followed by aromatisation through treatment with 2,6-lutidine leads to the title compounds in high yields. (C) 2000 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(00)01097-2
文献信息
-
An efficient method for the synthesis of 1-chlorophenazines based on the selective cathodic reduction of 3,3,6,6-tetrachloro-1,2-cyclohexanedione作者:Antonio Guirado、Alfredo Cerezo、Raquel Andreu、José I. López Sánchez、Delia BautistaDOI:10.1016/j.tet.2004.06.089日期:2004.8An efficient method for the synthesis of 1-chlorophenazines has been established. It is based on the use of 3,6,6-trichloro-2-hydroxy-2-cyclohexen-1-one 4 as a synthetic equivalent of 3-chloro-1,2-benzoquinone 3. The intermediate 4 was prepared in near quantitative yield by electroreductive monodechlorination of 3,3,6,6-tetrachloro-1,2-cyclohexanedione 1, which is an inexpensive and easily available已经建立了合成1-氯吩嗪的有效方法。它基于使用3,6,6-三氯-2-羟基-2-环己烯-1-酮4作为3-氯-1,2-苯醌3的合成等价物。通过对3,3,6,6-四氯-1,2-环己二酮1进行电还原单氯化,以接近定量的收率制备中间体4,这是一种廉价且易于获得的原料。4与伯1,2-苯二胺的有效反应提供了相应的1,1,4-三氯-1,2,3,4-四氢吩嗪6通过用2,6-二甲基吡啶处理直接将其芳香化,从而以高收率得到标题化合物。1,1,4-三氯-1,2,3,4-四氢-6-甲基吩嗪6f,8-苯甲酰基-1,1,4-三氯-1,2,3,4-四氢的X射线晶体学结构已确定了-phenazine 6ea和1,7-dichlorophenazine 10db。
-
One-Pot Synthesis of 1,4-Dichlorophenazines作者:Antonio Guirado、Alfredo Cerezo、M.Carmen Ramírez de ArellanoDOI:10.1016/s0040-4020(97)00275-5日期:1997.4An efficient, generally applicable one-pot method for the synthesis of 1,4-dichlorophenazines has been established. The method is based on the use of 3,3,6,6-tetrachloro-1,2-cyclohexanedione 2 as a synthetic equivalent of 3,6-dichloro-1,2-benzoquinone 1. The reaction of 2 with primary 1,2-arylidenediamines followed by treatment with pyridine provides the title compounds in nearly quantitative yields. 1,1,4,4-Tetrachloro-1,2,3,4-tetrahydrophenazines are isolable intermediates. The crystallographic X-ray structure of 7,7'-Bis(1,1,4,4-tetrachloro-1,2,3,4-tetrahydrophenazine) 7 has been determined. (C) 1997 Elsevier Science Ltd.
-
Rapid Bergman Cyclization of 1,2-Diethynylheteroarenes作者:Chang-Sik Kim、K. C. RussellDOI:10.1021/jo980879p日期:1998.11.1The synthesis and cyclization of acyclic quinoxaline, pyridine, and pyrimidine enediynes (1-3) are described. These compounds were prepared using palladium(0) coupling of trimethylsilyl acetylene to o-dihalo- or o-halotriflic heteroarenes. All compounds were prepared in modest to good yields. The enediynes prepared were shown to undergo Bergman cyclization. Kinetics over a minimum of 3 half-lives were used to construct Arrhenius plots. Pyrimidine 3 was found to have an activation energy of 16.1 kcal/mol. Cyclization of the closest known aromatic analogue, o-diethynylbenzene (15), has E-a = 25.1 kcal/mol (Grissom, J. W.; Calkins, T. L.;McMillen, H. A.; Jiang, Y. J. Org. Chem. 1994, 59, 5833-5835). Pyridine 2 and quinoxaline 1 gave activation energies of 21.5 and 33.6 kcal/mol, respectively. The results illustrate that heteroarenes can be used to activate Bergman cyclization. We expect these compounds to play an important role in furthering the understanding of Bergman cyclization and in aiding the development of new biologically significant enediynes.
-
Maffei et al., Gazzetta Chimica Italiana, 1953, vol. 83, p. 327,812作者:Maffei et al.DOI:——日期:——
-
Tschernezkii; Kiprianow, Zhurnal Obshchei Khimii, 1953, vol. 23, p. 1743,1749;engl.Ausg.S.1839,1843作者:Tschernezkii、KiprianowDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
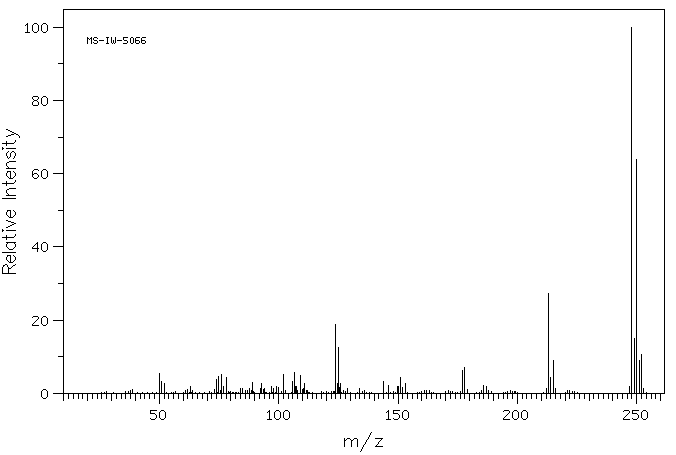
质谱MS
-
碳谱13CNMR
-
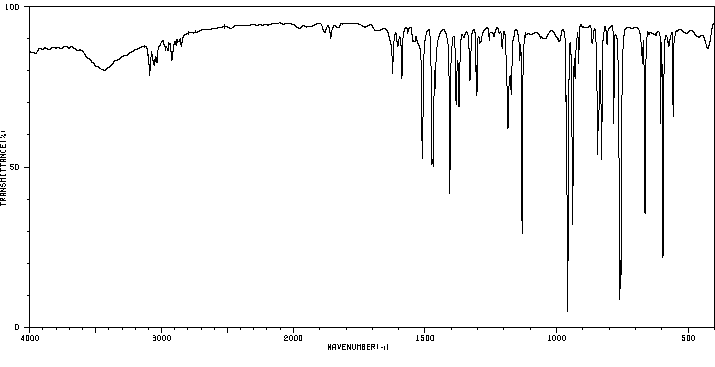
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮