4-戊烷-2-基苯胺 | 30273-14-4
中文名称
4-戊烷-2-基苯胺
中文别名
——
英文名称
(+/-)-4-(1-methyl-butyl)-aniline
英文别名
4-(1-methyl-butyl)-aniline;(+/-)-4-(1-Methyl-butyl)-anilin;4-(1-Methyl-butyl)-anilin;4-(2'-pentyl)aniline;Methyl-propyl-<4-amino-phenyl>-methan;4-<1-Methyl-butyl>-anilin;Benzenamine, 4-(1-methylbutyl)-;4-pentan-2-ylaniline
CAS
30273-14-4
化学式
C11H17N
mdl
——
分子量
163.263
InChiKey
AKSSDFIINGVZJQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.45
-
拓扑面积:26
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
反应信息
-
作为反应物:描述:4-戊烷-2-基苯胺 生成 4-仲戊基酚参考文献:名称:Revision of species of the “Oligia” semicana group (Lepidoptera: Noctuidae) with descriptions of a new genus and 12 new species摘要:AbstractThe name Neoligiagen.nov. is proposed for the "Oligia" semicana (Walker) species group in North America and 12 species, N. rubirena sp.nov., N. pagosa sp.nov., N. hardwicki sp.nov、N. inermis sp.nov.、N. invenusta sp.nov.、N. albirena sp.nov.、N. lancea sp.nov.、N. elephas sp.nov.、N. lillooet sp.nov.、N. surdirena sp.nov.、N. canadensis sp.nov.和 N. atlantica sp.nov.等 12 个种。Hadenella laevigata Smith 与 Hadena tonsa Grote 同源(同义恢复),Hadena hausta Grote 与 Miana semicana Walker 同源(同义恢复)。所有物种的成体以及已知的雄性和雌性生殖器均附有插图。DOI:10.4039/ent134157-2
-
作为产物:描述:参考文献:名称:Identification of Alkylbenzenes. II. Identification of the Eight Amylbenzenes and Cyclopentylbenzene by Means of Their Mono- and Diacetamino and Monobenzamino Derivatives摘要:DOI:10.1021/ja01273a060
文献信息
-
[EN] PROCESSES FOR THE PREPARATION OF NIRAPARIB AND INTERMEDIATES THEREOF<br/>[FR] PROCÉDÉS DE PRÉPARATION DE NIRAPARIB ET INTERMÉDIAIRES DE CELUI-CI申请人:TEVA PHARMA公开号:WO2019036441A1公开(公告)日:2019-02-21The present invention relates to novel procedures and novel intermediates useful in the synthesis of Niraparib or any salt thereof.本发明涉及用于合成尼拉帕尼布或其任何盐的新型程序和新型中间体。
-
Novel ortho-quinone diazide photoresist sensitizers申请人:RCA Corporation公开号:US04207107A1公开(公告)日:1980-06-10Ortho-quinone diazide compounds of the formula ##STR1## wherein R is an organic radical are useful sensitizers for photoresist compositions and intermediates for novel dyes.式为##STR1##其中R为有机基团的正醌二重氮化合物是光刻胶组合物的有用敏化剂,也是新型染料的中间体。
-
Catalytic Access to 4-(sec-Alkyl)Anilines via 1,6-Conjugate Addition of Grignard Reagents to <i>in Situ</i> Generated aza-<i>p</i>-Quinone Methides作者:Mercedes Zurro、Luo Ge、Syuzanna R. HarutyunyanDOI:10.1021/acs.orglett.2c02786日期:2022.9.16The synthesis of aniline derivatives, common building blocks in many pharmaceuticals, agrochemicals, dyes or polymers, has been limited to reactions based on benzene-toluene-xylene derivatives (BTX) due to their ample availability. Despite the large number of existing methodologies, the synthesis of chiral 4-(sec-alkyl)anilines has not been accomplished so far. In this work, a tandem strategy based
-
Crystalline polypropylene resin composition and amide compounds申请人:NEW JAPAN CHEMICAL CO.,LTD.公开号:EP0557721A2公开(公告)日:1993-09-01Disclosed are a crystalline polypropylene resin composition comprising a crystalline polyproplylene resin and a β-nucleating agent, and a method of increasing the proportion of β-form crystals in a crystalline polypropylene resin molding comprising molding the composition, the β-nucleating agent being a diamide compound.
-
Porous stretched article of polypropylene-based resin and process for its preparation申请人:NEW JAPAN CHEMICAL CO.,LTD.公开号:EP0632095A2公开(公告)日:1995-01-04Disclosed are a process for producing a porous stretched article of β-crystalline polypropylene-based resin, the process comprising the steps of crystallizing a melt of a polypropylene-based resin composition at a temperature of 15 to 140°C to form a solidified product containing β-form crystals and stretching the resulting solidified product at a temprature which is higher than 20 °C but lower than the melting point of the β-form crystals in said solidified product, the polypropylene-based resin composition comprising a polypropylene-based resin and 0.0001 to 5 parts by weight, per 100 parts by weight of the polypropylene-based resin, of at least one specific amide compound; and a porous stretched article produced by the process.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
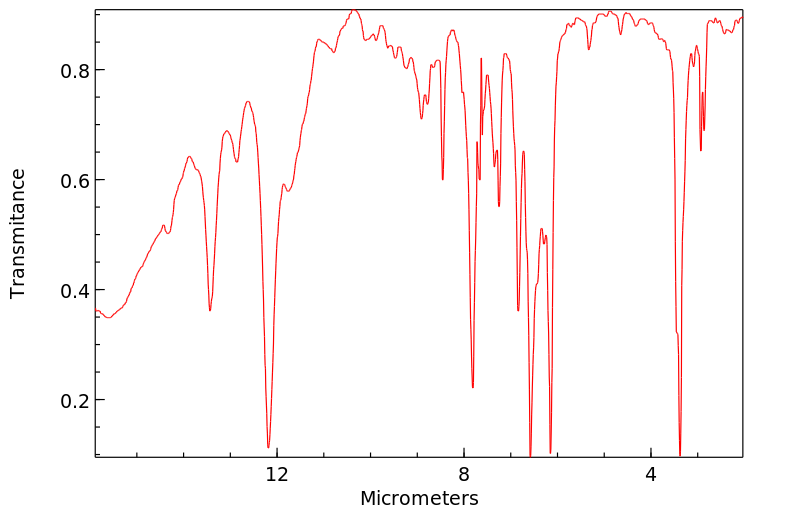
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫