l-2-异硫代氰酰基-4-(甲基硫代)丁酸甲酯 | 21055-47-0
中文名称
l-2-异硫代氰酰基-4-(甲基硫代)丁酸甲酯
中文别名
2-硫代异氰酸酯邻-4-(甲巯基)丁酸甲酯;2-硫代异氰酸邻-4-(甲基硫基)丁酸甲酯;2-硫代异氰酸邻-4-(甲巯基)丁酸甲酯
英文名称
2-isothiocyanato-4-methylsulfanyl butyric acid methyl ester
英文别名
methyl 2-isothiocyanato-4-(methylthio)butanoate;N-Thiocarbonyl-methionin-methylester;Methyl 2-isothiocyanato-4-(methylthio)butyrate;methyl 2-isothiocyanato-4-methylsulfanylbutanoate
CAS
21055-47-0
化学式
C7H11NO2S2
mdl
MFCD00060386
分子量
205.302
InChiKey
HNBACGFGPNFPAF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:112°C 1mm
-
密度:1.18
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:12
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:96
-
氢给体数:0
-
氢受体数:5
安全信息
-
危险等级:IRRITANT-HARMFUL
-
海关编码:2930909090
SDS
反应信息
-
作为反应物:描述:l-2-异硫代氰酰基-4-(甲基硫代)丁酸甲酯 在 四氯化钛 、 N,N-二异丙基乙胺 作用下, 以 二氯甲烷 为溶剂, 反应 0.5h, 以54%的产率得到DL-2,3-bis-(2-methylsulfanylethyl)-2,3-diisothiocyanato-succinic acid dimethyl ester参考文献:名称:钛(IV)介导的2,3-二异硫氰酸根合琥珀酸二酯和3,6-二硫代-哌嗪衍生物的合成摘要:2-异硫氰酸根合-羧酸酯的钛(IV)烯醇盐的氧化均偶联导致2,3-二异硫氰酸根合-琥珀酸二酯的合成。反应是使用DIPEA / TiCl 4氧化系统进行的,并以手性二聚体(而非内消旋体)为主要产物。衍生自受阻2-异硫氰酸根合羧酸盐的钛(IV)烯醇盐未经历氧化均偶联,但得到了3,6-二硫代-哌嗪。DOI:10.1016/j.tet.2007.03.053
-
作为产物:描述:DL-蛋氨酸甲酯盐酸盐 在 sodium persulfate 、 potassium carbonate 作用下, 以 水 为溶剂, 反应 1.0h, 生成 l-2-异硫代氰酰基-4-(甲基硫代)丁酸甲酯参考文献:名称:Na 2 S 2 O 8介导的水中伯胺高效合成异硫氰酸酯摘要:我们已经开发了两种绿色,实用且有效的方法,包括一锅法,可通过胺和二硫化碳通过胺合成异硫氰酸酯。用过硫酸钠脱硫。使用水作为溶剂。为了使异硫氰酸酯具有良好的化学选择性,必须具备碱性条件。通过两种方法,结构令人满意的直链和支链烷基胺和芳基胺很容易以令人满意的产率转化为异硫氰酸酯。卤素,苄基CH键,甲硫基,硝基,酯,烯基,富电子或不足的(杂)芳基,乙炔基,甚至酚和醇羟基均被很好地耐受。在水中一锅法也可用于从手性胺制备手性异硫氰酸酯,以及用游离氨基修饰生物活性结构。在大规模制备中,开发了独立于柱色谱法的简单实用的纯化方法。DOI:10.1039/c8gc02261e
文献信息
-
A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur作者:R. Nickisch、P. Conen、S. M. Gabrielsen、M. A. R. MeierDOI:10.1039/d0ra10436a日期:——Isothiocyanates (ITCs) are typically prepared using amines and highly toxic reagents such as thiophosgene, its derivatives, or CS2. In this work, an investigation of a multicomponent reaction (MCR) using isocyanides, elemental sulfur and amines revealed that isocyanides can be converted to isothiocyanates using sulfur and catalytic amounts of amine bases, especially DBU (down to 2 mol%). This new catalytic异硫氰酸酯 (ITC) 通常使用胺和高毒性试剂(例如硫光气、其衍生物或 CS2)来制备。在这项工作中,对使用异氰化物、元素硫和胺的多组分反应 (MCR) 的研究表明,可以使用硫和催化量的胺碱,特别是 DBU(低至 2 mol%)将异氰化物转化为异硫氰酸盐。这种新的催化反应在可持续性方面进行了优化,特别是考虑到在适度加热 (40 °C) 下使用 Cyrene™ 或 γ-丁内酯 (GBL) 等良性溶剂。进一步优化柱色谱纯化,通过保持产品的高纯度来减少废物的产生。因此,E 因子低至 0.989,并且通过在催化条件下转化 20 种不同的异氰化物,同时获得中等到高产率 (34-95%),显示了这种简单程序的多功能性。
表征谱图
-
氢谱1HNMR
-
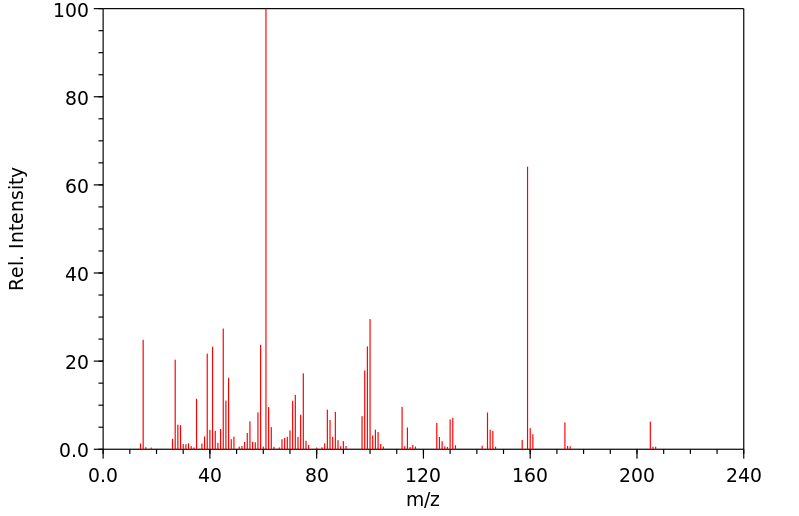
质谱MS
-
碳谱13CNMR
-
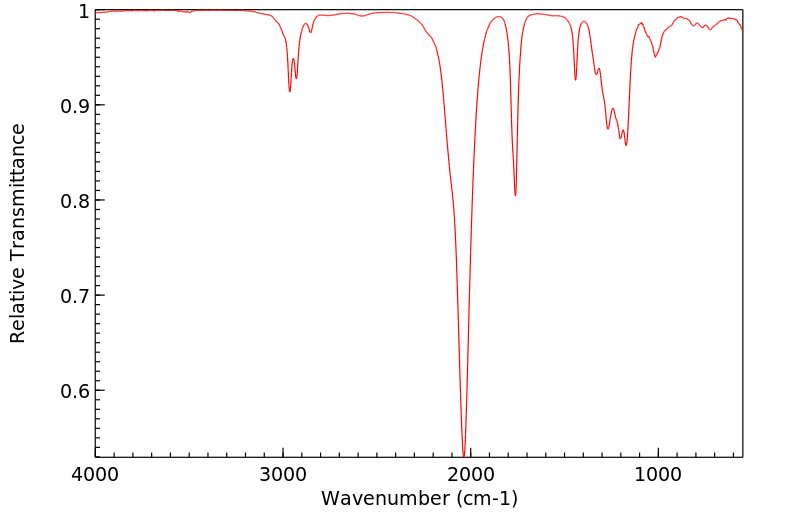
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸