2-(4-溴苯基二甲基硅基)苯甲醇 | 947515-73-3
中文名称
2-(4-溴苯基二甲基硅基)苯甲醇
中文别名
——
英文名称
(4-bromophenyl)[2-(hydroxymethyl)phenyl]dimethylsilane
英文别名
{2-[(4-Bromophenyl)dimethylsilyl]phenyl}methanol;[2-[(4-bromophenyl)-dimethylsilyl]phenyl]methanol
CAS
947515-73-3
化学式
C15H17BrOSi
mdl
——
分子量
321.289
InChiKey
XUQDWNXYJIOKBY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:55.1-55.8 °C
-
沸点:379.9±42.0 °C(Predicted)
-
密度:1.31±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.76
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (4-Bromo-phenyl)-dimethyl-[2-(tetrahydro-pyran-2-yloxymethyl)-phenyl]-silane 953412-90-3 C20H25BrO2Si 405.407
反应信息
-
作为反应物:描述:参考文献:名称:有机[2-(羟甲基)苯基]二甲基硅烷作为用于铑催化的 1,4-加成反应的温和且可重复的试剂摘要:稳定且可重复使用的四有机硅试剂、烯基-、芳基-和甲硅烷基[2-(羟甲基)苯基]二甲基硅烷,在温和的铑催化下与 α,β-不饱和羰基受体发生 1,4-加成反应。该反应耐受多种官能团,适用于克级合成。手性二烯配体的使用允许使用四有机硅试剂实现相应的对映选择性转化,为用作药物合成中间体的光学活性酮和取代哌啶酮提供基于硅的方法。根据观察到的对映选择性 1 中的外消旋手性苯基硅烷的动力学分辨率,建议铑醇盐物种负责金属转移步骤,DOI:10.1021/ja071969r
-
作为产物:描述:1,4-二溴苯 、 1,3-二氢-1,1-二甲基-2,1-苯并氧硅杂环戊二烯 在 正丁基锂 作用下, 以 正己烷 、 四氢呋喃 为溶剂, 反应 2.0h, 以91%的产率得到2-(4-溴苯基二甲基硅基)苯甲醇参考文献:名称:有机[2-(羟甲基)苯基]二甲基硅烷作为用于铑催化的 1,4-加成反应的温和且可重复的试剂摘要:稳定且可重复使用的四有机硅试剂、烯基-、芳基-和甲硅烷基[2-(羟甲基)苯基]二甲基硅烷,在温和的铑催化下与 α,β-不饱和羰基受体发生 1,4-加成反应。该反应耐受多种官能团,适用于克级合成。手性二烯配体的使用允许使用四有机硅试剂实现相应的对映选择性转化,为用作药物合成中间体的光学活性酮和取代哌啶酮提供基于硅的方法。根据观察到的对映选择性 1 中的外消旋手性苯基硅烷的动力学分辨率,建议铑醇盐物种负责金属转移步骤,DOI:10.1021/ja071969r
文献信息
-
Copper-Catalyzed Electrophilic Amination of Arylsilanes with Hydroxylamines作者:Yuya Miki、Koji Hirano、Tetsuya Satoh、Masahiro MiuraDOI:10.1021/ol303222s日期:2013.1.4A copper-catalyzed electrophilic amination of aryl[(2-hydroxymethyl)phenyl]dimethylsilanes with O-acylated hydroxylamines has been developed to afford the corresponding anilines in good yields. The catalytic reaction proceeds very smoothly under mild conditions and tolerates a wide range of functional groups.
-
Rhodium-catalyzed Addition of Organo[2-(hydroxymethyl)phenyl]dimethylsilanes to Arenesulfonylimines作者:Yoshiaki Nakao、Masahide Takeda、Jinshui Chen、Tamejiro Hiyama、Yoshitaka Ichikawa、Ryo Shintani、Tamio HayashiDOI:10.1246/cl.2008.290日期:2008.3.5The title reaction is found to proceed in the presence of a rhodium/diene catalyst. Variously substituted diarylmethylamines and allylamines having an N-arenesulfonyl protection are obtained in good yields, which are important building blocks in organic synthesis.
-
A Silicon-Based Approach to Oligoarenes by Iterative Cross-Coupling Reactions of Halogenated Organo[(2-hydroxymethyl)phenyl]dimethylsilanes作者:Yoshiaki Nakao、Jinshui Chen、Masaaki Tanaka、Tamejiro HiyamaDOI:10.1021/ja074728s日期:2007.9.1Highly efficient synthesis of silyl-substituted oligoarenes with defined structures is achieved by an iterative cross-coupling reaction and deprotection sequence using organo[(2-hydroxymethyl)phenyl]dimethylsilanes. The excellent stability of the tetraorganosilicon compounds as well as mild and divergent conditions for cross-coupling and deprotection steps allows preparation of highly conjugated oligoarenylsilanes such as unsymmetrically disilylated quinquethiophenes.
表征谱图
-
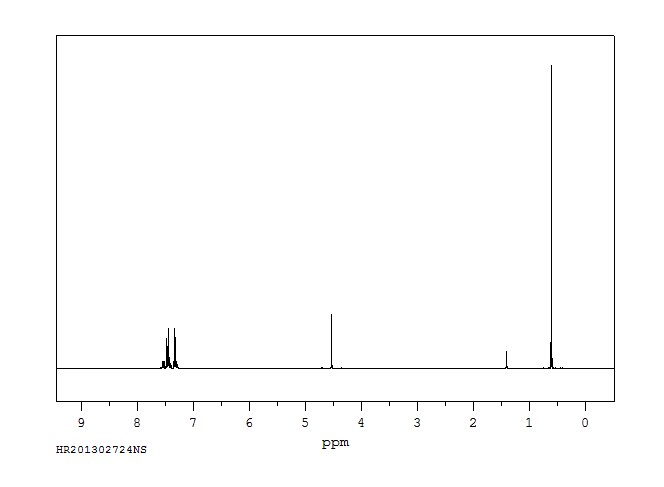
氢谱1HNMR
-
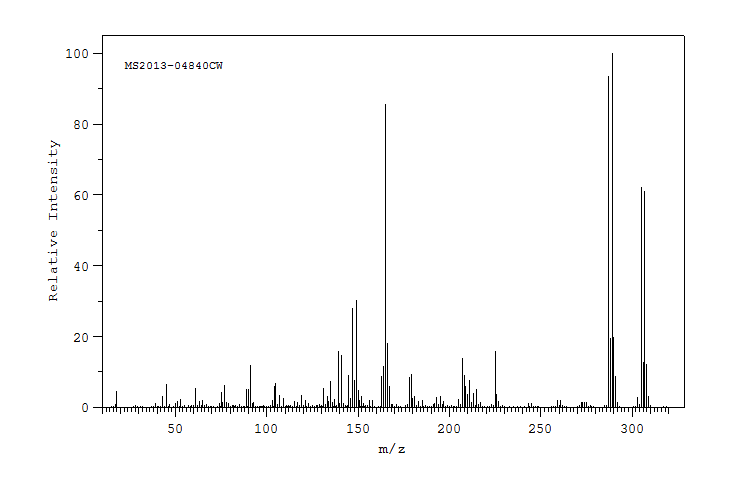
质谱MS
-
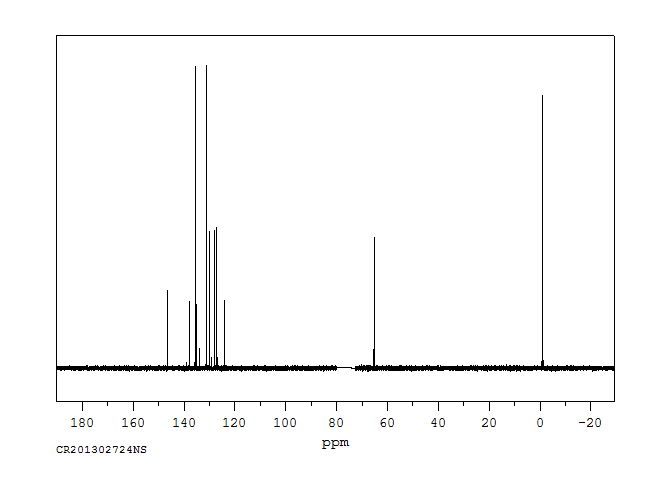
碳谱13CNMR
-
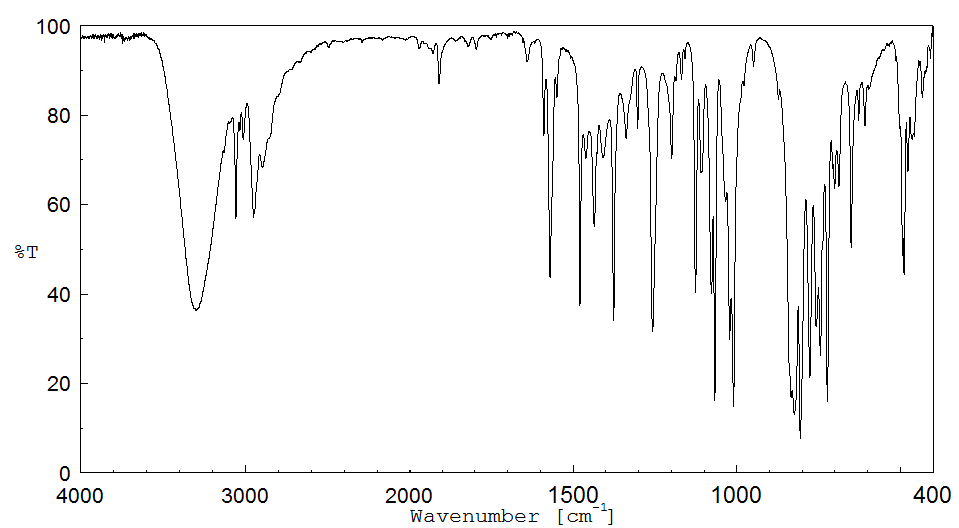
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫