ortho-phthalic acid bis(trimethylsilyl) ester | 2078-22-0
中文名称
——
中文别名
——
英文名称
ortho-phthalic acid bis(trimethylsilyl) ester
英文别名
phthalic acid bis(trimethylsilyl)ester;bis(trimethlsilyl) ortho-phthalate;bis(trimethylsilyl) phthalate;1,2-Benzenedicarboxylic acid, bis(trimethylsilyl) ester;bis(trimethylsilyl) benzene-1,2-dicarboxylate
CAS
2078-22-0
化学式
C14H22O4Si2
mdl
——
分子量
310.497
InChiKey
JUZOFKLPPHUUMC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1675;1675
计算性质
-
辛醇/水分配系数(LogP):3.67
-
重原子数:20
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.43
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 邻苯二甲酸 benzene-1,2-dicarboxylic acid 88-99-3 C8H6O4 166.133
反应信息
-
作为反应物:描述:ortho-phthalic acid bis(trimethylsilyl) ester 在 三甲基氯硅烷 作用下, 以70%的产率得到4,7-bis(trimethylsilyl)isobenzofuran-1,3-dione参考文献:名称:双-邻-metalation的未受保护的/甲硅烷基化ø -邻氨基酸。直接获得新的甲硅烷基化的N-羟基邻苯二甲酰亚胺(NHPI)类似物摘要:所述一个锅,原位邻-metalation /不受保护的甲硅烷基化ø -邻羧酸使用四甲基哌啶锂(LiTMP)和三甲基氯进行说明。该方法可以直接获得甲硅烷基化的邻苯二甲酸酐。这些可以容易地转化成相应的甲硅烷基化的N-羟基邻苯二甲酰亚胺(NHPI)类似物,它们是有前途的新型好氧氧化催化剂。DOI:10.1016/j.tetlet.2011.10.114
-
作为产物:参考文献:名称:双-邻-metalation的未受保护的/甲硅烷基化ø -邻氨基酸。直接获得新的甲硅烷基化的N-羟基邻苯二甲酰亚胺(NHPI)类似物摘要:所述一个锅,原位邻-metalation /不受保护的甲硅烷基化ø -邻羧酸使用四甲基哌啶锂(LiTMP)和三甲基氯进行说明。该方法可以直接获得甲硅烷基化的邻苯二甲酸酐。这些可以容易地转化成相应的甲硅烷基化的N-羟基邻苯二甲酰亚胺(NHPI)类似物,它们是有前途的新型好氧氧化催化剂。DOI:10.1016/j.tetlet.2011.10.114
文献信息
-
Synthesis of trimethylsilyl carboxylates by HMDS under solvent-free conditions作者:Marjan Jereb、Janja LaknerDOI:10.1016/j.tet.2016.08.003日期:2016.9carboxylic acids were transformed into their trimethylsilyl esters with HMDS in a practically completely solvent-free process, while a catalytic amount of iodine was required in some cases. The process has several advantages over the known methods: untreated reactants, air atmosphere, mild and neutral conditions, no evolution of hydrogen halide, no need of an additional base, low amount of waste, completely
-
Silacyclophanones 3*. Cyclic organosilicon esters of ortho-phthalic acids作者:Sergey V. Basenko、Anastasiya S. Soldatenko、Aleksandr V. Vashchenko、Vladimir I. SmirnovDOI:10.1007/s10593-019-02592-5日期:2019.11bis(trimethylsilyl) ester of ortho-phthalic acid (1:1, 20°C, 168–264 h) in hexane lead to the formation of previously unknown 14-membered cyclic organosilicon esters of ortho-phthalic acids (silacyclophanones) in 36–85% yields. Molecular structures of 14-membered cyclic ethers 4,10-dimethyl-4,10-diphenyl-3,5,9,11-tetraoxo-4,10-disyla-1,7(1,2)-dibenzacyclododecaphane-2,6,8,12-tetraone, 4,4,10,10-tetramethyl-3在没有溶剂的情况下,二氯二甲基硅烷与2,3,4,5-四氟邻苯二甲酸双(三甲基硅烷基)醚的反应(1:1,20°C,24 h),二氯(氯甲基)甲基硅烷和二氯(甲基)苯基硅烷与双(三甲基硅烷基)的反应)邻苯二甲酸的邻苯二甲酸酯(1:1,20°C,168–264 h)在己烷中的形成导致以前未知的邻苯二甲酸的14元环状有机硅酸酯(硅环环酮)的形成率为36–85% 。14元环醚4,10-二甲基-4,10-二苯基-3,5,9,11-四氧代-4,10-二甲苯基1,7(1,2)-二苯甲环十二碳六烯2,6的分子结构,8,12-tetraone,4,4,10,10-tetramethyl-3,5,9,11-tetraoxo-4,10-disila-1,7(1,2)-di-(tetrafluorobenza)cyclododecaphane-2 ,6,8,12-丁酮,以及2,3,4,5-四氟邻苯二甲酸的双(三甲基
-
Silacyclophanones – cyclic organosilicon esters of isophthalic acid作者:Sergey V. Basenko、Anastasiya S. SoldatenkoDOI:10.1007/s10593-018-2239-5日期:2018.1of previously unknown 16-membered cyclic organosilicon esters of isophthalic acid (silacyclophanones) in 69–77% yields. The reaction of isophthalic ester with 1,3-dichloro-1,3-dimethyl-1,3-divinyldisiloxane gave 14-membered cyclosiloxane ester as the main product, while the reaction of disiloxane with trimethylsilyl ester of phthalic acid produced a 9-membered cyclic ester in 65% yield.
-
Basenko, S. V.; Bormashev, P. A.; Mirskov, R. G., Doklady Chemistry, 1993, vol. 331, # 1-3, p. 173 - 175作者:Basenko, S. V.、Bormashev, P. A.、Mirskov, R. G.、Voronkov, M. G.DOI:——日期:——
-
Basenko S. W., Bormashew P. A., Mirskow R. G., Woronkow M. G., Dokl. AN, 331 (1993) N 2, S 177-178作者:Basenko S. W., Bormashew P. A., Mirskow R. G., Woronkow M. G.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
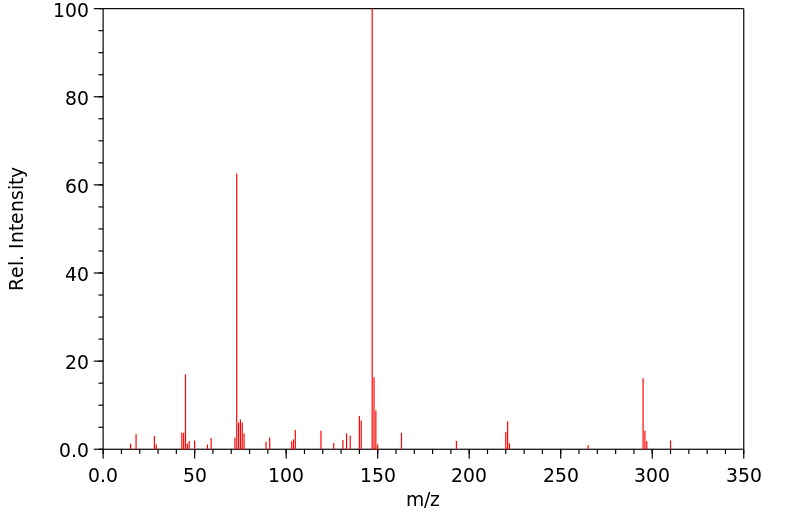
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫