(3R)-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolanyl)-4-methylpent-1-ene
中文名称
——
中文别名
——
英文名称
(3R)-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolanyl)-4-methylpent-1-ene
英文别名
CH2CHCH(isopropyl)BO2(C(CH3)2)2;4,4,5,5-tetramethyl-2-[(3R)-4-methylpent-1-en-3-yl]-1,3,2-dioxaborolane
CAS
——
化学式
C12H23BO2
mdl
——
分子量
210.124
InChiKey
ZIFKTLLUMRIEHF-JTQLQIEISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.29
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙烯基硼酸频哪醇酯 pinacol vinylboronate 75927-49-0 C8H15BO2 154.017
反应信息
-
作为反应物:参考文献:名称:Highly Diastereo- and Enantioselective Allylboration of Aldehydes using α-Substituted Allyl/Crotyl Pinacol Boronic Esters via in Situ Generated Borinic Esters摘要:Readily available, alpha-substituted allyl/crotyl pinacol boronic esters often give low E/Z selectivity (with Z favored) in reactions with aldehydes. We found that addition of nBuLi to the pinacol boronic ester followed by trapping of the alkoxide with TFAA leads to an intermediate allyl borinic ester which undergoes allylboration with very high E selectivity. The substrate scope includes primary to tertiary alkyl alpha-substituents, crotyl substrates, and the previously unreported beta-methallyl pinacol boronic esters. The latter give very high Z selectivity under standard conditions which is completely reversed to high E selectivity under the new conditions. Monitoring the reaction by B-11 NMR confirmed that the reaction proceeds through a borinic ester intermediate.DOI:10.1021/ja401564z
-
作为产物:描述:2-chloromethylvinylboronic acid pinacol ester 、 异丙基溴化镁 在 C4H3S(2-CO2Cu) 、 (S,S)-((S)-C20H12O2)PN(CH(CH3)C6H4(o-OCH3))2 作用下, 以 二氯甲烷 为溶剂, 生成 (3R)-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolanyl)-4-methylpent-1-ene参考文献:名称:α-取代的烯丙基硼酸酯的催化对映选择性制备:向功能化醛中一锅加成,以及制备手性烯丙基三氟硼酸酯试剂的途径。摘要:DOI:10.1002/anie.200700975
表征谱图
-
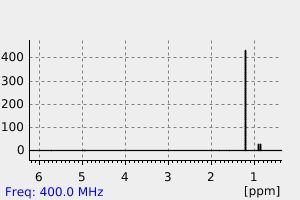
氢谱1HNMR
-
质谱MS
-
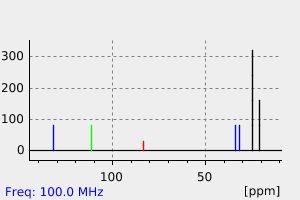
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-三甲基甲硅烷基)-乙氧基甲基三氟硼酸钾
频哪醇(二氯甲基)硼酸酯
顺式-2-丁烯-1-硼酸频那醇酯
钾环丙基甲基三氟硼酸
钾反-1-癸烯基三氟硼酸
钾三氟(戊基)硼酸酯(1-)
钾三氟(丙基)BORANUIDE
钾三氟(1-己炔-1-基)硼酸酯(1-)
钾1-癸炔-1-基(三氟)硼酸酯(1-)
钾(E)-丙烯基-1-三氟硼酸
钾(E)-丙烯基-1-三氟硼酸
钾(2-甲氧基乙基)三氟硼酸酯
辛基硼酸频呢醇酯
辛基三氟硼酸钾
羟基二异丙基硼烷
羟基二丙基硼烷
碘甲基硼酸频哪醇酯
硼酸频那醇异丁酯
硼酸,二甲基,甲酯
硼酸,(4-溴丁基)-,二甲基酯
硼烷胺,N,1-二溴-N-(1,1-二甲基乙基)-1-甲基-
硼烷胺,1-溴-N-(1,1-二甲基乙基)-1-乙基-
硼烷,二氯(1-甲基乙烯基)-
甲氧基甲基硼酸
甲氧基甲基三氟硼酸钾
甲基硼酸频呐醇酯
甲基硼酸新戊二醇酯
甲基硼酸-d3
甲基硼酸
甲基双(二异丙基氨基)硼烷
甲基二环戊基硼酸酯
甲基二氯硼烷
甲基二己基硼酸酯
甲基二丁基硼酸酯
甲基三氟硼酸钾
甲基7-甲氧基苯并噻吩-2-羧酸酯
甲基2-(4-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)环己-3-烯基)乙酸甲酯
甲基-硼酸二甲酯
环戊烷三氟硼酸钾
环戊烯-1-基硼酸
环戊氧基甲基三氟硼酸钾
环戊基硼酸频呢醇酯(含有数量不等的酸酐)
环戊基硼酸-1,3-丙二醇酯
环戊基硼酸
环庚烯-1-基硼酸
环庚基硼酸
环庚基三氟硼酸钾
环己酮-3-硼酸酯
环己烷硼酸频那醇酯
环己烯基三氟硼酸钾