2-溴-4-甲基苯基硫代异氰酸酯 | 19241-39-5
中文名称
2-溴-4-甲基苯基硫代异氰酸酯
中文别名
2-溴-4-甲基苯异硫氰酸酯
英文名称
(2-bromo-4-methylphenyl)isothiocyanate
英文别名
2-Bromo-4-methylphenyl isothiocyanate;2-bromo-1-isothiocyanato-4-methylbenzene
CAS
19241-39-5
化学式
C8H6BrNS
mdl
——
分子量
228.112
InChiKey
DQXSCOWMTPPFCJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险类别码:R20/21/22,R36/37/38
-
危险品运输编号:2810
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
-
安全说明:S26,S36/37/39
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-溴-4-甲基苯胺 2-bromo-p-toluidine 583-68-6 C7H8BrN 186.051 对甲苯异硫氰酸酯 1-isothiocyanato-4-methylbenzene 622-59-3 C8H7NS 149.216
反应信息
-
作为反应物:描述:参考文献:名称:Muthusamy, Sengoden; Paramasivam, Rangasamy; Ramakrishnan, Vayalakkavoor T., Journal of Heterocyclic Chemistry, 1991, vol. 28, # 3, p. 759 - 763摘要:DOI:
-
作为产物:描述:triethylamine (2-bromo-4-methylphenyl)carbamodithioate 在 碘 、 三乙胺 作用下, 以 乙酸乙酯 为溶剂, 生成 2-溴-4-甲基苯基硫代异氰酸酯参考文献:名称:一锅制备 氰胺 使用分子碘从二硫代氨基甲酸酯中提取摘要:公开了一种通过使用分子碘的双脱硫策略由二硫代氨基甲酸盐合成氰胺的有效的一锅法。二硫代氨基甲酸酯,通过碘的作用异硫氰酸酯 原位,经水处理NH 3 给 硫脲。这硫脲因此生成的产物经I 2进一步氧化脱硫,从而以良好的收率得到相应的氰酰胺。环境友好性,成本效益和高产量是这一一锅法的重要属性。DOI:10.1039/b914283p
文献信息
-
Synthesis and Octopaminergic-agonist Activity of 3-(Substituted Phenyl)imidazolidine-2-thiones and Related Compounds作者:Akinori HIRASHIMA、Kenji SHINKAI、Eiichi KUWANO、Eiji TANIGUCHI、Morifusa ETODOI:10.1271/bbb.62.1179日期:1998.13-(Substituted phenyl)imidazolidine-2-thiones (SPITs) and related compounds were synthesized by cyclizing monoethanolamine hydrogen sulfate with arylisothiocyanates in the presence of sodium hydroxide. The activity for stimulating adenylate cyclase prepared from thoracic nerve cords of the American cockroach, Periplaneta americana L., was examined with these compounds. A SPIT with a 2,6-diethylphenyl通过在氢氧化钠存在下将单乙醇胺硫酸氢盐与芳基异硫氰酸酯环化,可以合成3-(取代的苯基)咪唑烷-2-硫酮(SPIT)和相关化合物。用这些化合物检查了刺激从美国蟑螂美洲大i的胸神经线制备的腺苷酸环化酶的活性。具有2,6-二乙基苯基基团的SPIT(48)是唯一的完全激动剂,其他SPIT衍生物是部分激动剂。更大的酶活化似乎是由短链烷基而不是SPITs芳香环的2,6-位上的卤素取代引起的。在2,6-二取代的SPIT中,从甲基到乙基的链长增加导致酶激活增加。同时,在2中,从乙基到异丙基的链长进一步增加 6-二取代的SPIT导致酶活化降低。能量最小的章鱼胺和48的叠加显示出结构和构象相似,这说明48的Vmax值更高。在有效SPIT的咪唑烷环的C4或C5烷基化后,酶的活化作用明显降低。因此,在SPIT的苯环和N-末端的2-位和6-位的一定程度的蓬松度和疏水性对于活化腺苷酸环化酶是有利的。
-
Substituted Spiro Compounds and Their Use for Producing Pain-Relief Medicaments申请人:Frank Robert公开号:US20080269271A1公开(公告)日:2008-10-30The present invention relates to substituted spiro compounds, to processes for preparing them, to medicaments comprising these compounds and to the use of these compounds for producing medicaments.本发明涉及替代螺环化合物,涉及制备这些化合物的方法,涉及含有这些化合物的药物以及利用这些化合物生产药物的用途。
-
Integrated One-Flow Synthesis of Heterocyclic Thioquinazolinones through Serial Microreactions with Two Organolithium Intermediates作者:Heejin Kim、Hyune-Jea Lee、Dong-Pyo KimDOI:10.1002/anie.201410062日期:2015.2.2thioquinazolinone derivatives are synthesized within 10 s in high yields (75–98 %) at room temperature. These three‐step reactions involve two organolithium intermediates, an isothiocyanate‐functionalized aryllithium intermediate, and a subsequent lithium thiolate intermediate. We also demonstrate the gram‐scale synthesis of a multifunctionalized thioquinazolinone in the microfluidic device with a high yield通过微反应器中的短寿命中间体合成药物化合物具有吸引力,因为它具有快速流动和高通量的特点。另外,可以顺序利用中间体以在短时间内有效地建立文库。在这里,我们提出了生物活性硫喹唑啉酮文库的集成微流合成。一代Ø通过在室温下将微反应器中的停留时间控制在16 ms,可以优化异硫氰酸-硫代苯硫基酯和随后与异氰酸芳基酯的反应。在室温下,可以在10 s内以高收率(75–98%)合成各种S-苄基硫代喹唑啉酮衍生物。这三步反应涉及两个有机锂中间体,一个异硫氰酸酯官能化的芳基锂中间体,以及随后的硫醇锂中间体。我们还演示了在微流体装置中以克级合成多官能硫代喹唑啉酮的方法,该方法具有较高的收率(91%)和生产率(5分钟内为1.25g)。
-
An “on-water” exploration of CuO nanoparticle catalysed synthesis of 2-aminobenzothiazoles作者:Saroj Kumar Rout、Srimanta Guin、Jayashree Nath、Bhisma K. PatelDOI:10.1039/c2gc35575b日期:——An âon-waterâ one-pot process has been engineered for the preparation of 2-aminobenzothiazole from ortho-halo (âF, âCl, âBr and âI) substituted unsymmetrical thioureas. For ortho âI and âBr substrates the reactions afford 2-aminobenzothiazoles under metal free condition promoted by base. However, the relatively inert ortho âCl and âF substrates undergo intramolecular arylthiolation only in the presence of CuO nanoparticles yielding 2-aminobenzothiazoles. This methodology provides easy access to aminobenzothiazoles utilising even the ortho âCl and âF substrates. The catalyst is recyclable several times without loss of substantial activity. Other remarkable features include the wide range of functional group tolerance, absence of chromatographic purification (for ortho âI and âBr substrates) and providing moderate to excellent yield of the products under mild conditions, thus rendering the methodology as a highly eco-friendly alternative to the existing methods.
-
Copper(I)-catalyzed cascade reaction of 2-haloaryl isothiocyanates with isocyanides: a strategy to construct benzo[d]imidazo[5,1-b]thiazoles作者:Wenyan Hao、Xiaoyan Sang、Jing Jiang、Mingzhong CaiDOI:10.1016/j.tetlet.2016.02.084日期:2016.3A copper(I) catalyzed cascade reaction of 2-haloaryl isothiocyanates with isocyanides for the construction of benzo[d]imidazo[5,1-b]thiazoles has been demonstrated. Good to excellent yields could be achieved. This [3+2] cycloaddition and C–S coupling reaction represents an extremely simple way to construct benzo[d]imidazo[5,1-b]thiazoles.
表征谱图
-
氢谱1HNMR
-
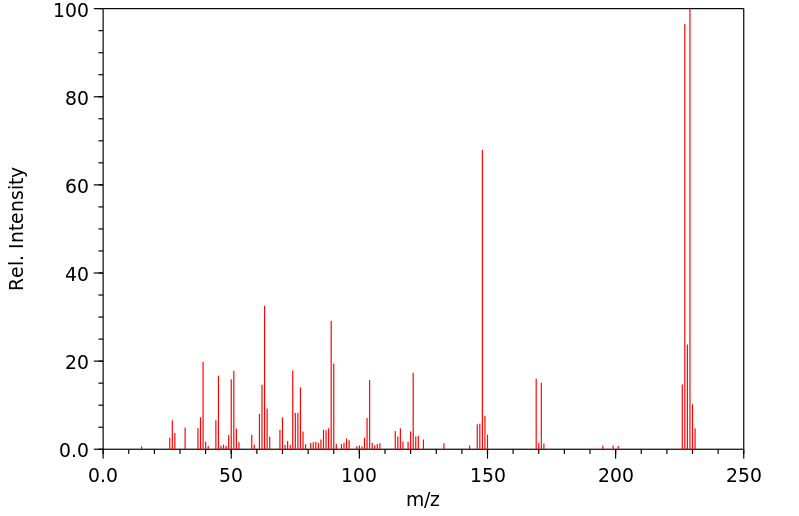
质谱MS
-
碳谱13CNMR
-
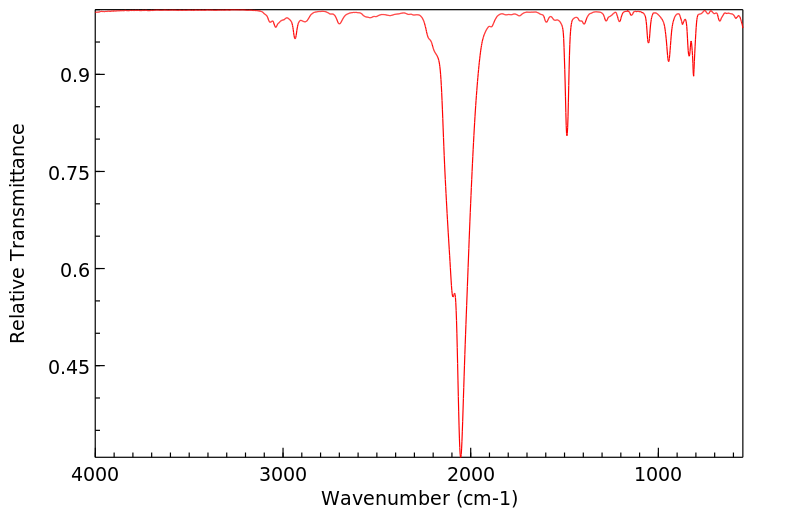
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫