4-羟基-2-疏基蝶啶 | 52023-48-0
中文名称
4-羟基-2-疏基蝶啶
中文别名
——
英文名称
4-oxo-2-thioxo-1,2,3,4-tetrahydropteridine
英文别名
1,2-dihydro-2-thioxopteridin-4(3H)-one;2-thioxo-2,3-dihydropteridin-4(1H)-one;2-thiopteridine;2-thiolumazine;4-thiolumazine;2-thioxo-2,3-dihydro-1H-pteridin-4-one;2-sulfanylidene-1H-pteridin-4-one
CAS
52023-48-0
化学式
C6H4N4OS
mdl
MFCD00023899
分子量
180.19
InChiKey
VSPCYUNOZPCHLL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:99
-
氢给体数:2
-
氢受体数:4
安全信息
-
海关编码:2933990090
SDS
| Name: | 4-Hydroxy-2-Mercaptopteridine Material Safety Data Sheet |
| Synonym: | 2-Mercapto-4-Pteridino |
| CAS: | 52023-48-0 |
Synonym:2-Mercapto-4-Pteridino
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 52023-48-0 | 4-Hydroxy-2-Mercaptopteridine | 100.0 | 257-612-8 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Mercaptans may cause nausea and headache. Exposure to high concentrations of mercaptans can produce unconsciousness with cyanosis (bluish discoloration of skin due to deficient oxygenation of the blood), cold extremities and rapid pulse.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Exposure to high concentrations of mercaptans can produce unconsciousness with cyanosis (bluish discoloration of skin due to deficient oxygenation of the blood), cold extremities and rapid pulse. Mercaptans may cause nausea and headache.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use only in a chemical fume hood.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 52023-48-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white to off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: > 300 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H4N4OS
Molecular Weight: 180.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 52023-48-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-Hydroxy-2-Mercaptopteridine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 52023-48-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 52023-48-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 52023-48-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:4-羟基-2-疏基蝶啶 在 碘 、 sodium hydroxide 作用下, 以 甲醇 、 氯仿 为溶剂, 反应 120.0h, 生成 9-iodo-10-phenyl-9,10-dihydro-5H,8H-[1,3]thiazino[3,2-a]pteridin-5-one参考文献:名称:熔融嘧啶体系:XVI。2-(烯基硫烷基)pteridin-4(3 H)-ones的亲电分子内环化摘要:2- [烯丙基(丁-3-烯-1-基,戊-4-烯-1-基)硫]蝶啶-4(3 Н) -酮在多磷酸加热经历分子内环化,得到分别9-甲基-8,9-二氢-5 H- [1,3]噻唑并[3,2 - a ]蝶啶-5-酮和10-甲基(乙基)-9,10-二氢-5Н,8Н- [1 ,3] thiazino [3,2- a ] pteridin-5-ones的角结构。的2-(alkenylsulfanyl)环化嘌呤-4-(3 Н) -酮碘的作用下或芳基亚磺酰基氯化物,得到碘(芳基硫基)噻唑融熔和thiazinopteridines的角度衍生物。DOI:10.1134/s1070428016050225
-
作为产物:描述:N-ethoxycarbonyl-N'-(3-benzyloxycarbonylpyrazinyl-2)-thiourea 在 sodium ethanolate 作用下, 以 乙醇 为溶剂, 反应 0.5h, 以48%的产率得到4-羟基-2-疏基蝶啶参考文献:名称:Koren, Bozidar; Stanovnik, Branko; Tisler, Miha, Heterocycles, 1987, vol. 26, # 3, p. 689 - 697摘要:DOI:
文献信息
-
Immunosuppressive effects of pteridine derivatives申请人:——公开号:US20030236255A1公开(公告)日:2003-12-25Novel poly-substituted pteridinediones (lumazines), and mono- or polysubstituted 2-thiolumazines, 4-thiolumazines or 2,4-dithiolumazines, having disclosed substituents in positions 1, 3, 6 and 7 of the pteridine ring, and pharmaceutically acceptable salts thereof, are useful as biologically active ingredients in preparing pharmaceutical compositions especially for the treatment or prevention of a CNS disorder, a cell proliferative disorder, a viral infection, an immune or auto-immune disorder or a transplant rejection. Combinations of the pteridine derivatives of the invention with an immunosuppressant or immunomodulator drug, an antineoplastic drug or an antiviral agent, providing potential synergistic effects, are also disclosed.
-
AFFINITY ILLUDOFULVENE CONJUGATES申请人:AF Chemicals, LLC,公开号:US20210155583A1公开(公告)日:2021-05-27In an embodiment of the invention, a composition for treating a cell population comprises a medicant. The medicant moiety can be an illudofulvene analog. In an embodiment of the invention, a composition for treating a cell population comprises an Affinity Medicant Conjugate (AMC). The affinity moiety can be an antibody, an antibody fragment, a receptor protein, a peptidic growth factor, an anti-angiogenic protein, a specific binding peptide, protease cleavable peptide, a glycopeptide, a peptide, a peptidic toxin, a protein toxin and an oligonucleotide. The affinity moiety can be covalently bound to the medicant via a linker.
-
Gold(I) Complexes Incorporating Emissive Mercapto‐Pteridine Ligands: Syntheses, X‐ray Structure, Luminescence and Preliminary Cytotoxic Evaluation作者:Lucy A. Mullice、Huw J. Mottram、Andrew J. Hallett、Simon J. A. PopeDOI:10.1002/ejic.201200147日期:2012.6The syntheses of six new mixed P/S-donor two-coordinate AuI complexes are described. The complexes incorporate a pteridinyl ligand coordinated through a thiolate donor, and an ancillary tertiary phosphane (PPh3 or PCy3). The mercapto-pteridine ligands (L1–L3) differ in the nature of the substituents on the pteridine core. An X-ray crystal structure was obtained for one of the examples, [(L1)Au(PPh3)]描述了六种新的混合 P/S 供体双坐标 AuI 配合物的合成。该复合物包含通过硫醇盐供体和辅助叔膦(PPh3 或 PCy3)配位的蝶啶基配体。巯基蝶啶配体 (L1-L3) 的不同之处在于蝶啶核上取代基的性质。获得了其中一个实例 [(L1)Au(PPh3)] 的 X 射线晶体结构,揭示了复合物的两个分子之间的弱分子间相互作用:芳环之间的 π-π 接触似乎支持分子间 Au-Au大约 3.05 A 的接触。所有的复合物在溶液中都是发光的,发射来自可调谐的基于配体的激发态,并且本质上是一种扰动的荧光。在这种情况下,L3 的复合物显示出有用的可见光吸收和发射。使用 MTT 测定法进行了初步细胞毒性评估,对于四种不同的腺癌细胞系(MCF7、A549、PC3 和 LOVO),每种复合物都显示出令人印象深刻的抗增殖活性(IC50 < 5 μm)。对于给定的蝶啶部分,三苯基膦似乎是增强生物活性的首选配体。
-
A Novel Synthesis of 1,2,4-Triazolopteridines作者:Tayseer Abdallah、Manal Darwish、Hamdi HassaneenDOI:10.3390/70600494日期:——Reaction of 1,2-dihydro-2-thioxopteridin-4(3H)-one, 6,7-dimethyl-1,2-dihydro-2-thioxopteridin-4(3H)-one and 6,7-diphenyl-1,2-dihydro-2-thioxopteridin-4(3H)-one with hydrazonoyl halides affords the title compounds in good yields.
-
Pyranopterin Dithiolene Distortions Relevant to Electron Transfer in Xanthine Oxidase/Dehydrogenase作者:Chao Dong、Jing Yang、Silke Leimkühler、Martin L. KirkDOI:10.1021/ic500873y日期:2014.7.21band yields resonance Raman spectra of the Mo site that are devoid of contributions from the highly absorbing FAD and 2Fe2S clusters in the protein. The resonance Raman spectra reveal in-plane bending modes of the bound product and low-frequency molybdenum dithiolene and pyranopterin dithiolene vibrational modes. This work provides keen insight into the role of the pyranopterin dithiolene in electron-transfer
表征谱图
-
氢谱1HNMR
-
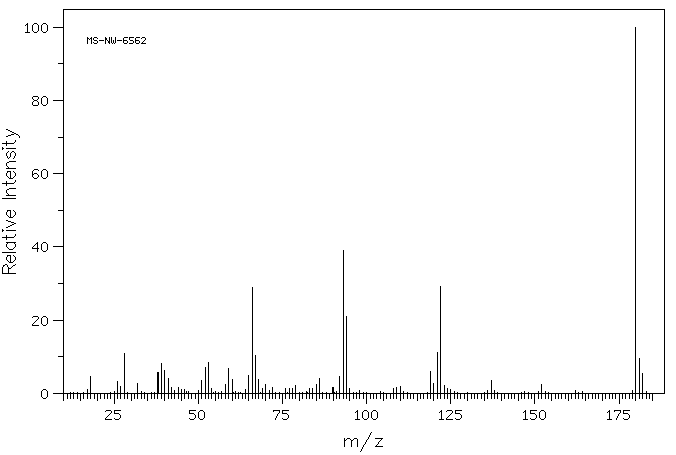
质谱MS
-
碳谱13CNMR
-
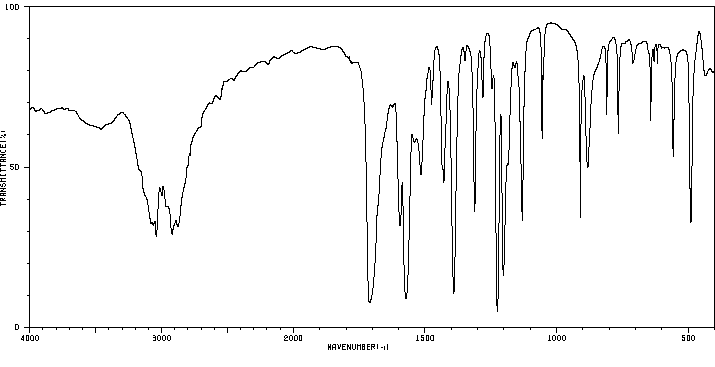
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄素酰色氨酸
高蝶酸
骏河毒素
酵母粉
诺米林酸17-β-D-吡喃葡萄糖苷
蝶酸
蝶啶3-氧化物
蝶啶-6-基-甲醇
蝶啶-4,6-二胺
蝶啶-2,4-二胺
蝶呤-6-羧酸
苯癸酸,2-羟基-3,4-二甲氧基-6-甲基
苯并[g]蝶啶-4a(2H)-基,5-乙基-3,4,5,10-四氢-3,7,8,10-四甲基-2,4-二羰基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,5-乙酰基-5,10-二氢-1,3-二甲基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,5,10-二氢-7,8-二甲基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,1,7,8-三甲基-
羧甲基黄素
羟基-2-吡啶酮
维生素 B2
维他命 B2
硫酸氢3-(6,7-二氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N-乙基-N-(2-羟基乙基)丙烷-1-铵
硫酸氢2-(7,8-二氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N,N-二甲基乙铵
甲氨蝶呤钠
甲氨蝶呤杂质1
生物蝶呤-d3
生物喋呤中间体
环己烯,3-氟-4-(甲硫基)-,反-(9CI)
玫瑰黄色素
溴化氢溴化1-(2-氨基乙基)-3-甲基-4-[(Z)-2-萘-1-基乙烯基]吡啶正离子
氯化3-(7-氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N,N-二甲基丙烷-1-铵
氨蝶呤钠
氨苯蝶啶
氨甲酸,[(1S)-2-羟基-1-甲基丙基]-,1,1-二甲基乙基酯(9CI)
氨甲蝶呤
氨基蝶呤
核黄素还原
核黄素杂质Q
核黄素5'-硫酸盐
核黄素3′,4′-二磷酸酯
核黄素-4'-磷酸
核黄素-3'-磷酸盐
核黄素,2',3',4',5'-四乙酸酯
核黄素 5'-丁酸酯
核黄素
无色喋呤
异黄蝶呤
己二酸,2-[[4-[[(2-氨基-1,4,5,6,7,8-六氢-4-羰基-6-蝶啶基)甲基]氨基]苯甲酰]氨基]-
左亚叶酸钙杂质
左亚叶酸钙
四氢蝶酰五谷氨酸酯