1-(二甲氨基)-2-硝基乙烯 | 73430-27-0
分子结构分类
中文名称
1-(二甲氨基)-2-硝基乙烯
中文别名
——
英文名称
(E)-N,N-dimethyl-2-nitroethenamine
英文别名
(E)-N,N-dimethyl-2-nitroethen-1-amine;1-dimethylamino-2-nitroethylene;N,N-dimethyl-2-nitroethenamine
CAS
73430-27-0
化学式
C4H8N2O2
mdl
——
分子量
116.12
InChiKey
JKOVQYWMFZTKMX-ONEGZZNKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-0.8
-
重原子数:8
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:49.1
-
氢给体数:0
-
氢受体数:3
安全信息
-
包装等级:III
-
危险类别:8
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险品运输编号:1759
-
危险性描述:H302,H315,H317,H318,H335
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-1-Methylamino-2-nitroethene 86602-50-8 C3H6N2O2 102.093
反应信息
-
作为反应物:描述:1-(二甲氨基)-2-硝基乙烯 以64%的产率得到参考文献:名称:BUECHI G.; MAK C.-P., J. ORG. CHEM.
, 1977, 42, NO 10, 1784-1786 摘要:DOI: -
作为产物:描述:以 二氯甲烷 为溶剂, 以197 mg的产率得到1-(二甲氨基)-2-硝基乙烯参考文献:名称:硝酸甲硅烷基酯与二甲基甲酰胺二甲基乙缩醛的反应作为合成2-亚硝胺的新通用方法摘要:从相应的脂肪族硝基化合物就地获得的甲硅烷基磺酸盐与二甲基甲酰胺二甲基乙缩醛反应,以中等至良好的收率得到2-亚硝基胺。讨论了反应途径。DOI:10.1016/j.tetlet.2014.09.071
文献信息
-
IRAK DEGRADERS AND USES THEREOF申请人:Kymera Therapeutics, Inc.公开号:US20190192668A1公开(公告)日:2019-06-27The present invention provides compounds, compositions thereof, and methods of using the same.本发明提供了化合物、其组合物以及使用这些化合物的方法。
-
Total Synthesis of (±)-Decursivine作者:Andrew B. Leduc、Michael A. KerrDOI:10.1002/ejoc.200600922日期:2007.1The first preparation of the antimalarial natural product decursivine is described. A Diels–Alder/Plieninger indolization protocol allows convenient preparation of the indole 15 which, in turn is a suitable substrate for a boron–enolate aldol reaction with piperonal (16). The resulting adduct 14 is transformed efficiently to the natural product. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim,
-
KIO<sub>3</sub>-Catalyzed Aerobic Cross-Coupling Reactions of Enaminones and Thiophenols: Synthesis of Polyfunctionalized Alkenes by Metal-Free C–H Sulfenylation作者:Jie-Ping Wan、Shanshan Zhong、Lili Xie、Xiaoji Cao、Yunyun Liu、Li WeiDOI:10.1021/acs.orglett.5b03608日期:2016.2.5The synthesis of polyfunctionalized aminothioalkenes has been realized via the direct C–H sulfenylation of enaminones and analogous enamines. These cross-coupling reactions have been achieved by simple KIO3 catalysis under aerobic conditions without employing any transition metal catalyst or additional oxidant. The employment of bio-based green solvent ethyl lactate as the reaction medium constitutes
-
[EN] CARBOLINE CARBOXAMIDE COMPOUNDS USEFUL AS KINASE INHIBITORS<br/>[FR] COMPOSÉS DE CARBOLINE-CARBOXAMIDE UTILES EN TANT QU'INHIBITEURS DE KINASES申请人:BRISTOL MYERS SQUIBB CO公开号:WO2011159857A1公开(公告)日:2011-12-22Compounds having formula (I), and enantiomers, and diastereomers, stereoisomers, pharmaceutically-acceptable salts thereof, formula (I) are useful as kinase modulators, including Btk modulation.
-
Palladium-Catalyzed C-S Bond Formation of Stable Enamines with Arene/Alkanethiols: Highly Regioselective Synthesis of β-Amino Sulfides作者:Yaojia Jiang、Gaohui Liang、Cong Zhang、Teck-Peng LohDOI:10.1002/ejoc.201600588日期:2016.7A direct and regiocontrolled thiolation method to access β-amino sulfides through the palladium-catalyzed C(sp2)–H functionalization of stable enamines was described. The reaction was realized under mild conditions by adding an external phosphine ligand to prevent poisoning of the palladium catalyst by the sulfuric reagents. A possible mechanism was proposed according to the obtained results. The DFT
表征谱图
-
氢谱1HNMR
-
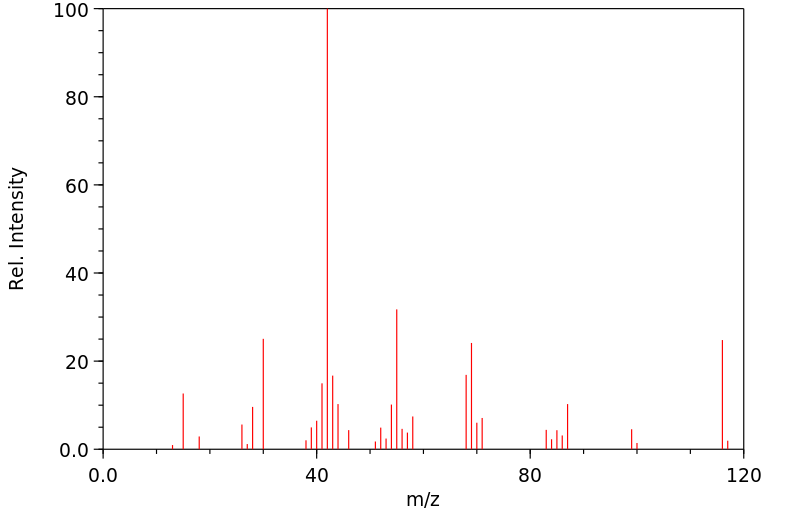
质谱MS
-
碳谱13CNMR
-
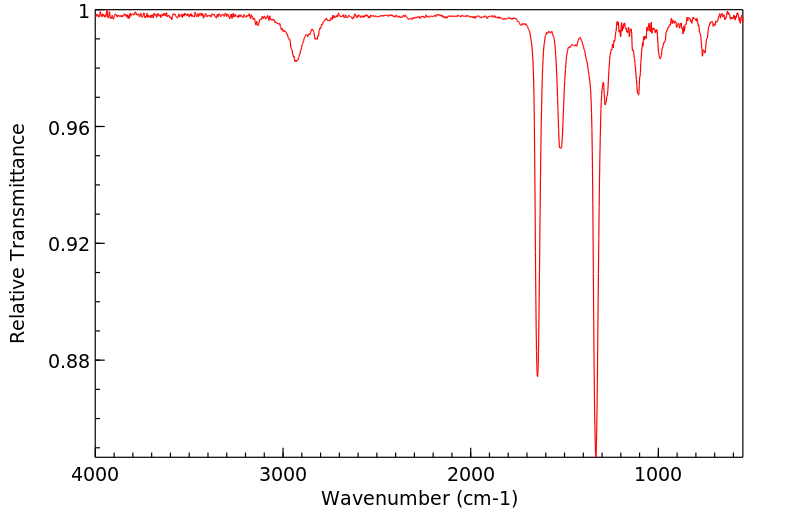
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-硝基环己基乙酸酯
顺式-2-硝基-6-甲基环己酮
雷尼替丁杂质18
铝硝基甲烷三氯化物
钾离子载体III
重氮(硝基)甲烷
醛基-七聚乙二醇-叠氮
过氧亚甲基
辛腈,4-氟-4-硝基-7-羰基-
辛烷,1,2-二氯-1-硝基-
赤霉素A4+7(GA4:GA7=65:35)
苄哒唑
羟胺-四聚乙二醇-叠氮
羟胺-三乙二醇-叠氮
米索硝唑
磷酸十二醇酯
碘硝基甲烷
碘化e1,1-二甲基-4-羰基-3,5-二(3-苯基-2-亚丙烯基)哌啶正离子
硝酰胺
硝基脲银(I)复合物
硝基甲醇
硝基甲烷-d3
硝基甲烷-13C,d3
硝基甲烷-13C
硝基甲烷-(15)N
硝基甲烷
硝基甲基甲醇胺
硝基环辛烷
硝基环戊烷
硝基环戊基阴离子
硝基环庚烷
硝基环己烷锂盐
硝基环己烷钾盐
硝基环己烷
硝基环丁烷
硝基氨基甲酸
硝基新戊烷
硝基二乙醇胺
硝基乙醛缩二甲醇
硝基乙醛缩二乙醇
硝基乙腈
硝基乙烷-D5
硝基乙烷-1,1-d2
硝基乙烷
硝基乙烯
硝基丙烷
硝基丙二醛(E,E)-二肟
硝基丙二腈
硝基-(3-硝基-[4]吡啶基)-胺
硝乙醛肟