N,N-二甲基乙硫胺 | 6338-68-7
中文名称
N,N-二甲基乙硫胺
中文别名
——
英文名称
Ethanesulfonic acid N,N-dimethylamide
英文别名
N,N-dimethylethanesulfonamide;ethanesulfonic acid dimethylamide;N,N-dimethyl-ethanesulfonamide;N,N-Dimethyl-aethansulfonamid;Aethansulfonsaeure-dimethylamid;N,N-dimethyl-ethanesulfonamide
CAS
6338-68-7
化学式
C4H11NO2S
mdl
MFCD00043617
分子量
137.203
InChiKey
MORLSCQKZAPYFM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-0.1
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2935009090
-
安全说明:S24/25
-
储存条件:应存于室温下,密封保存,并保持干燥。
SDS
反应信息
-
作为反应物:参考文献:名称:α-Substituted sulfonamides. II. α-Nitroso (oximino) derivatives摘要:DOI:10.1016/s0040-4039(01)87808-4
-
作为产物:描述:参考文献:名称:通过配体到金属电荷转移进行酰胺衍生物和醚的光诱导 C(sp3)–H 硫属化反应摘要:N-甲基酰胺和醚的光诱导、氯化铁(III)催化的C-H活化导致通过配体到金属电荷转移(LMCT)过程形成C-S和C-Se键。该方法以良好的收率将仲酰胺和叔酰胺、磺酰胺和氨基甲酸酯转化为相应的酰胺基-N,S-缩醛衍生物。机理工作表明,这种转变是通过涉及氯自由基中间体的氢原子转移(HAT)进行的。DOI:10.1021/acs.orglett.2c01505
文献信息
-
TETRACYCLIC CDK9 KINASE INHIBITORS申请人:AbbVie Inc.公开号:US20150218165A1公开(公告)日:2015-08-06Disclosed are compounds of Formula (Ia), and pharmaceutically acceptable salts thereof, wherein X, Y, R 1 , R 2 , R 3A , R 3B , and R 4 are as described herein. The compounds may be used as agents in the treatment of diseases, including cancer. Also disclosed are pharmaceutical compositions comprising one or more compounds of Formula (Ia).公开了公式(Ia)的化合物,以及其中X、Y、R1、R2、R3A、R3B和R4如本文所述的药用可接受盐。这些化合物可用作治疗疾病(包括癌症)的药物。还公开了包含一个或多个公式(Ia)化合物的药物组合物。
-
Stereoselective Synthesis of β-Substituted β-Amino Sulfones and Sulfonamides via Addition of Sulfonyl Anions to Chiral <i>N</i>-Sulfinyl Imines作者:Francisco Velázquez、Ashok Arasappan、Kevin Chen、Mousumi Sannigrahi、Srikanth Venkatraman、Andrew T. McPhail、Tze-Ming Chan、Neng-Yang Shih、F. George NjorogeDOI:10.1021/ol053132b日期:2006.2.1[reaction: see text] A highly stereoselective synthesis of beta-amino sulfones and sulfonamides via addition of sulfonyl anions to chiral N-sulfinyl imines is described. The addition reaction proceeds in good yield (75-99%) and stereoselectivity.
-
Catalytic alkylation reactions of weakly acidic carbonyl and related compounds using alkenes as electrophiles作者:Yasuhiro Yamashita、Ryo Igarashi、Hirotsugu Suzuki、Shū KobayashiDOI:10.1039/c8ob00941d日期:——Catalytic alkylation reactions of weakly acidic carbonyl and related pronucleophiles such as amides, esters, and sulfonamides with substituted alkenes have been reported. In the presence of a strong Brønsted base catalyst system, potassium hexamethyldisilazide and 18-crown-6 ether, the desired reactions proceeded in high yields at ambient temperature with a wide substrate scope. These are atom-economical
-
Catalytic Asymmetric Direct-Type 1,4-Addition Reactions of Alkanesulfonamides作者:Shū Kobayashi、Yasuhiro Yamashita、Ryo Igarashi、Hirotsugu SuzukiDOI:10.1055/s-0036-1588737日期:2017.74-addition reactions of alkanesulfonamides were developed on the ‘product base’ strategy. Alkanesulfonamides reacted with α,β-unsaturated amides in good to high yields with good to high diastereo- and enantioselectivities using a chiral alkaline metal amide. To our knowledge, this is the first example of a catalytic asymmetric C–C bond-forming reaction using an alkanesulfonamide without any activating group
-
Photochemical Regioselective C(sp<sup>3</sup>)–H Amination of Amides Using <i>N</i>-Haloimides作者:Lei Pan、Joseph Elmasry、Tomas Osccorima、Maria Victoria Cooke、Sébastien LaulhéDOI:10.1021/acs.orglett.1c00831日期:2021.5.7A metal-free regioselective C(sp3)–H amination of amides using N-haloimides in the presence of lithium tert-butoxide and visible light is presented herein. This photoexcited approach is straightforward, and it aminates a wide variety of amides under mild conditions without the use of photocatalysts, external radical initiators, or oxidants. A halogen-bonded intermediate between the tert-butoxide base
表征谱图
-
氢谱1HNMR
-
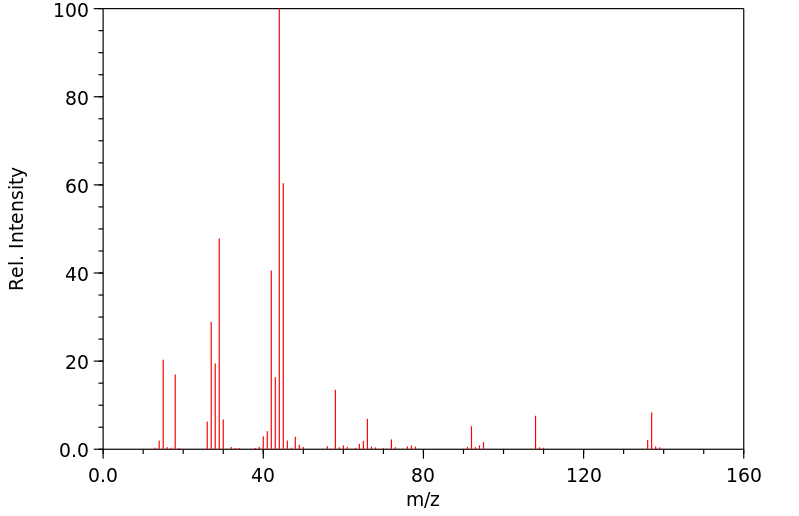
质谱MS
-
碳谱13CNMR
-
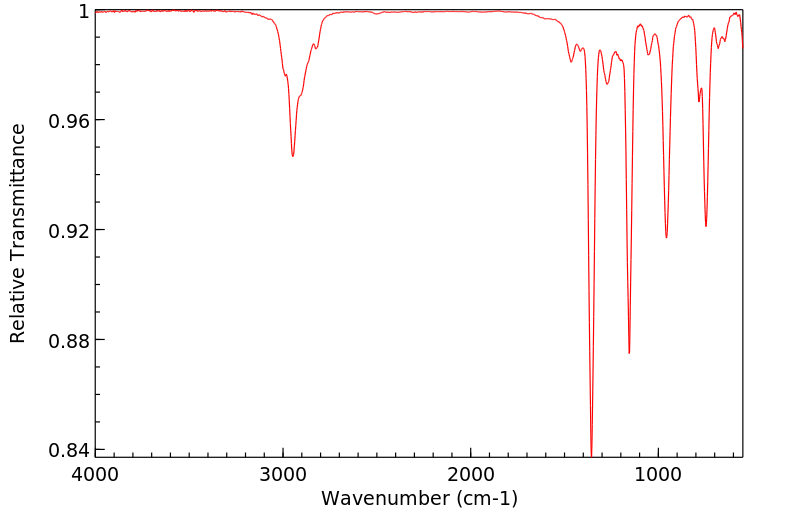
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高烯丙基(甲磺酰基)胺
高炔丙基(甲磺酰基)胺
顺式-9-十八碳烯基甲烷磺酸酯
顺式-4-乙基环己基甲烷磺酸酯
顺-1,2-双(甲磺酰基氧基甲基)环己烷
阿坎酸杂质
阿坎酸
锌甲烷磺酸盐
铵磺酸甜菜碱-3
铵磺酸甜菜碱-2
铵磺酸甜菜碱-1
铬雾抑制剂
铁三(三氟甲基磺酰基)亚胺
钾3-(三羟基硅烷基)-1-丙烷磺酸酯
钾1,1,2,2,3,3,4,4-八氟丁烷-1-磺酸盐
钡二乙烷磺酸酯
钠3-氨基丙烷磺酸酯
钠3-氨基-3-氧代-丙烷-1-磺酸酯
钠3-(三羟基硅烷基)-1-丙烷磺酸酯
钠2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-十八碳-9-烯氧基乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]-2-氧代-乙烷磺酸酯
钠1-羟基-1-庚烷磺酸酯
钠(1E)-1-十二碳烯-1-磺酸酯
酪朊酸钠
辛烷-1-磺酸甲酯
辛烷-1-磺酸乙酯
辛基-1-磺酸戊酯
辛-2-烯-1-磺酸
辅酶 M
西尼必利杂质7
萘-1,8-二甲醇
英丙舒凡对甲苯磺酸盐
英丙舒凡
苯基硒基三氟甲烷磺酸酯
芥酸酰胺丙基羟基磺基甜菜碱
艾日布林中间体
脒基牛磺酸
胺磺酸甜菜碱-4
联硫亚盐氯乙醛钠水合物
羧基-五聚乙二醇-磺酸
羟甲基磺酸钠
羟基甲烷磺酸铵盐
羟基甲烷磺酸钾
羟基甲氧基甲醇甲磺酸酯
羟乙磺酸钾
羟乙基磺酸铵盐
羟乙基磺酸钠
羟丙基硫代硫酸钠
美司那
磺酸钠
磺酸己烷