N'-(4-甲氧基苯基)-N,N-二甲基甲脒 | 1202-62-6
中文名称
N'-(4-甲氧基苯基)-N,N-二甲基甲脒
中文别名
——
英文名称
N'-(4-methoxyphenyl)-N,N-dimethylformimidamide
英文别名
N,N-Dimethyl-N'-p-methoxyphenyl-formamidin;N,N-Dimethyl-N'-(4-methoxy-phenyl)-formamidin;Methanimidamide, N'-(4-methoxyphenyl)-N,N-dimethyl-;N'-(4-methoxyphenyl)-N,N-dimethylmethanimidamide
CAS
1202-62-6
化学式
C10H14N2O
mdl
——
分子量
178.234
InChiKey
KEHWKNJZSFYKEM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1636;1636
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:24.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2925290090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲氧苯胺 4-methoxy-aniline 104-94-9 C7H9NO 123.155
反应信息
-
作为反应物:描述:参考文献:名称:Carbodiimide-sulfoxide reactions. XIII. Reactions of amines and hydrazine derivatives摘要:DOI:10.1021/jo00824a004
-
作为产物:描述:参考文献:名称:A Base Promoted Synthesis of N,N-dimethylformamidines摘要:DOI:10.5012/bkcs.2013.34.5.1537
文献信息
-
Change of the favored routes of EI MS fragmentation when proceeding from N1, N1-dimethyl-N2-arylformamidines to 1,1,3,3-tetraalkyl-2-arylguanidines: substituent effects作者:Ewa D. Raczyńska、Mariusz Makowski、Jean-François Gal、Pierre-Charles MariaDOI:10.1002/jms.1766日期:——ormamidines and of 1,1,3,3‐tetraalkyl‐2‐arylguanidines are structurally analogous and similar electron‐ionization mass spectral fragmentation may be expected, they display important differences in the favored routes of fragmentation and consequently in substituent effects on ion abundances. In the case of formamidines, the cyclization‐elimination process (initiated by nucleophilic attack of the N‐amino尽管N 1,N 1-二甲基N 2-芳基甲am和1,1,3,3-四烷基-2-芳基胍的系列在结构上相似,并且可能会出现类似的电离质谱碎片,但它们在有利的裂解途径,从而对离子丰度产生取代基效应。就甲am而言,环化消除过程(由苯环的2-位上的N-氨基原子的亲核攻击引发)和环状苯并咪唑鎓[M-H] +离子的形成占主导,而损失NR 2该组更优选胍。为了获得有关主要片段最可能结构的信息,对选定的一组进行了量子化学计算。线性对数之间关系的良好我[M-H] +我[M] +• }和σ - [R +在取代基常数对在苯环位置仅用于甲脒(发生- [R = 0.989)。在胍的情况下,这种关系并不重要(r = 0.659)。在log I [M‐NMe 2 ] + / I [M] +• }与σ之间发现良好的线性关系p +常数(r = 0.993)。版权所有©2010 John Wiley&Sons,Ltd.
-
Novel synthesis of 1-substituted-4-imidazolecarboxylates via solvent-free cycloaddition reaction between formamidines and isocyanides作者:Han Cao、Fu-sheng Bie、Xue-jing Liu、Ying Han、Jie Ma、Yi-jun Shi、Peng Yan、Chao-yue Sun、Hai-meng WangDOI:10.1016/j.tet.2020.131205日期:2020.5A simple and efficient protocol for cyclization between formamidines and ethyl isocyanoacetate has been described in the absence of metal catalyst and solvent. A series of 1-substituted-4-imidazolecarboxylates were synthesized in moderate to good yields with DABCO as base additive.
-
Amidines. Part 20. Rates of reaction of N,N-dialkylformamide acetals with substituted anilines作者:Jerzy Osek、Janusz Oszczapowicz、Witold DrzewińskiDOI:10.1039/p29860001961日期:——Rates of reaction of seven N,N-dialkylformamide acetals R12N–CH(OR2)2 with a series of anilines substituted on the phenyl ring have been measured in benzene, methanol, chloroform, and tetrahydrofuran by use of a g.l.c. method. In each case studied reaction is irreversible and obeys a second-order kinetic equation. Reaction rates correlate with Hammett σ constants for substituents on the phenyl ring
-
Cycloaddition von Heterocumulenen an C?N-Bindungssysteme. 1. Mitteilung. Perhydro-s-triazindione und Perhydro-s-triazinyl-harnstoffe aus N,N,N?-trisubstituieten Formamidinen und Isocyanaten作者:Karl SeckingerDOI:10.1002/hlca.19730560221日期:1973.1.31The addition of methyl isocyanate to N,N-dimethyl-N′-arylformamidines 4d–4r leads to the perhydro-s-triazine-diones 5d–5o and to the s-triazinylureas 10a–10k. The mechanism of formation is discussed.加入甲基异氰酸酯与N,N-二甲基-N'- arylformamidines图4d - 4R通向全氢小号嗪二酮图5d - 50和到小号-triazinylureas 10A - 10K。讨论了形成机理。
-
Formation of Amidines from Aryliminodimagnesium and<i>N</i>,<i>N</i>-Dimethylformamide. Novel Catalytic Mediation by Single Electron Transfer with Use of Nitrobenzenes作者:Masao Okubo、Mikio Tanaka、Koji MatsuoDOI:10.1246/cl.1990.1005日期:1990.6N,N′-Diarylformamidines were obtained in good yields by the reaction of aryliminodimagnesium (ArN(MgBr)2) with N,N-dimethylformamide in the presence of nitrobenzene. Novel catalytic mediation by single electron transfer was disclosed.
表征谱图
-
氢谱1HNMR
-
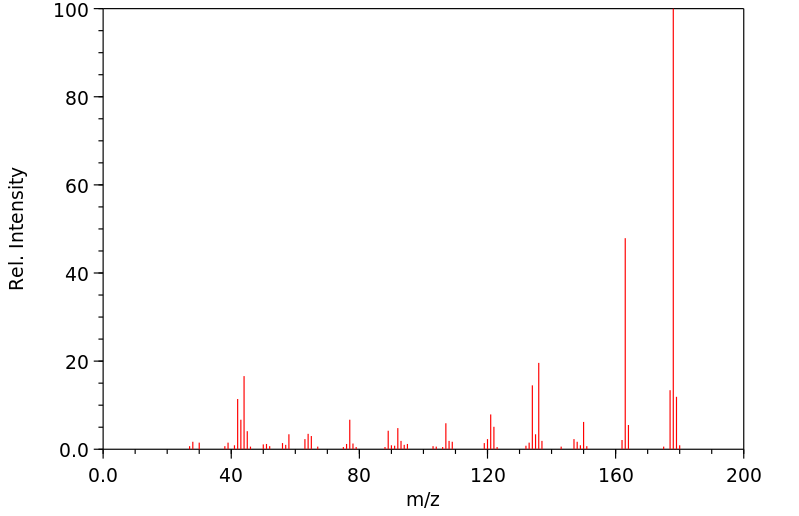
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫