3-二乙氨基-2,2-二甲基丙醛 | 6343-47-1
中文名称
3-二乙氨基-2,2-二甲基丙醛
中文别名
——
英文名称
3-diethylamino-2,2-dimethyl-propionaldehyde
英文别名
3-N,N-diethylamino-2,2-dimethylpropanal;3-(diethylamino)-2,2-dimethylpropanal;3-diethylamino-2,2-dimethylpropanal;3-Diaethylamino-2,2-dimethyl-propionaldehyd;Diaethylamino-pivalinaldehyd;3-Diethylamino-2,2-dimethyl-propionaldehyd
CAS
6343-47-1
化学式
C9H19NO
mdl
MFCD00043601
分子量
157.256
InChiKey
AAHIYAKUVPMLMX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
溶解度:可溶于氯仿(少许)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:11
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.89
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2922399090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-diethylamino-2,2-dimethylpropylamine 1000-17-5 C9H22N2 158.287
反应信息
-
作为反应物:描述:参考文献:名称:Preparation of aminoalcohols摘要:公开号:US02477842A1
-
作为产物:描述:参考文献:名称:Mannich; Wieder, Chemische Berichte, 1932, vol. 65, p. 385,389摘要:DOI:
文献信息
-
METHOD OF STABILIZING IMINO-FUNCTIONAL SILANE申请人:Momentive Performance Materials Inc.公开号:US20180016287A1公开(公告)日:2018-01-18A method of stabilizing imino-functional silane involving adding thereto at least one Brønsted-Lowry base to inhibit, suppress or prevent the addition reactions of the imino-functional silane with itself to form a imino- and amino-functional silane and the subsequent deamination reactions to form conjugated carbon-carbon double bond-containing imino-functional silanes and stabilized imino-functional silanes containing the at least one Brønsted-Lowry base.
-
Preparation of α-substituted acroleins via the reaction of aldehyde or the corresponding ozonide with dihalomethane and diethylamine作者:Yung-Son Hon、Feng-Jon Chang、Ling Lu、Wei-Chi LinDOI:10.1016/s0040-4020(98)00216-6日期:1998.5Treatment of aldehydes or the corresponding ozonides with a mixture of dibromomethane and diethylamine afforded α-substituted acroleins in modest to good yields. The β-carbon of the acrolein (nc, n = 1–16) derived from dibromomethane. Functional groups, such as ketone, hydroxy, acetoxy, bromide, iodide, ester are compatible with this reaction condition. The relative rates and yields of this transformation
-
Synthesis of Dialkyl [1-Hydroxy-2,2-dimethyl-3-(dialkylamino)propyl]phosphonates作者:M. B. Gazizov、R. A. Khairullin、L. P. Bagauva、O. G. Sinyashin、L. G. Gaisin、R. F. Karimova、R. M. ShaikhutdinovDOI:10.1023/b:rugc.0000016007.14621.11日期:2003.9We found that compounds III can be prepared by reaction of dialkyl hydrogen phosphites I with 3-(dialkylamino)-substituted aldehydes II. Addition of ethanolic sodium ethylate or a little sodium to a mixture of compounds I and II gives rise to an exothermic reaction. After neutralization of the reaction mixture with acetic acid and evaporation of volatile substances in a high vacuum, compounds III were
-
SECONDARY AMINOSILANES申请人:SIKA TECHNOLOGY AG公开号:US20130281562A1公开(公告)日:2013-10-24The present disclosure invention relates to novel secondary aminosilanes, a method for producing same, and the use thereof. The secondary aminosilanes can be produced from readily available reactants in a simple manner. The secondary aminosilanes are characterized for example by a low viscosity and are well suited for producing silane-functional polymers that have a low viscosity, fast curing, and good thermal stability.
-
ALDIMINES AND COMPOSITIONS COMPRISING ALDIMINE申请人:Burckhardt Urs公开号:US20100101455A1公开(公告)日:2010-04-29The present invention relates in a first consideration to aldimines of formula (I). Said aldimines are particularly suitable as latent hardeners in curable compositions, particularly compositions comprising one or two-component isocyanate groups. Due to the presence of the reactive group, they can be converted to compounds of the formulas (X) and (XII) comprising aldimine groups, implementing a further consideration of the present invention. The aldimines, or the compounds comprising aldimine groups, can be used primarily in adhesive and sealant materials, but also in coatings, and can be produced easily from readily available source materials, and have good thermal stability. The tertiary amino group thereof has a surprisingly low alkalinity and can in some cases have a catalytic effect in chemical reaction systems.
表征谱图
-
氢谱1HNMR
-
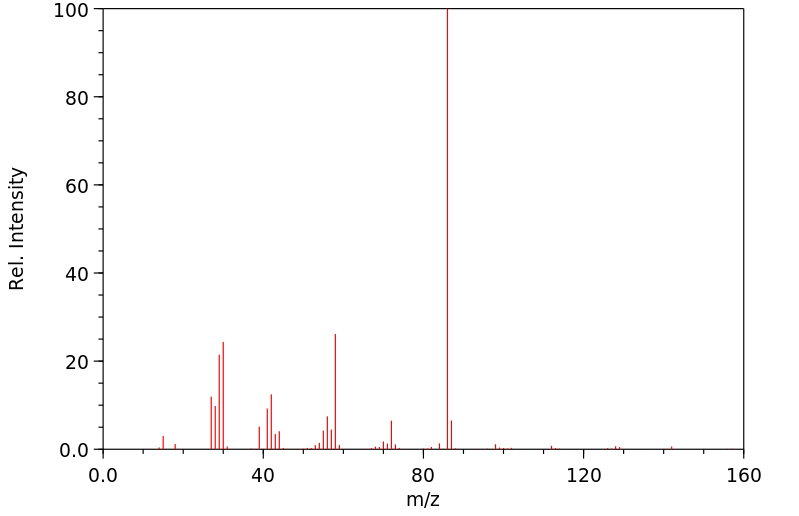
质谱MS
-
碳谱13CNMR
-
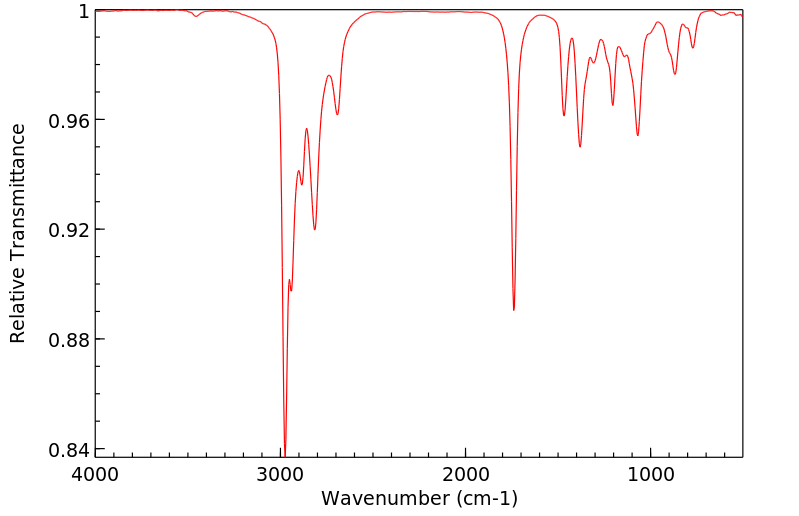
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷