1,3-辛二醇 | 23433-05-8
中文名称
1,3-辛二醇
中文别名
氨甲酸,[(1R,4S)-4-羟基-2-环戊烯-1-基]-,1,1-二甲基乙基
英文名称
1,3-octane diol
英文别名
octane-1,3-diol;1,3-octanediol;1,3-octandiol
CAS
23433-05-8
化学式
C8H18O2
mdl
MFCD01711163
分子量
146.23
InChiKey
DCTMXCOHGKSXIZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:68-69℃
-
沸点:165-170℃ (25 Torr)
-
密度:0.9636 (rough estimate)
-
LogP:1.298 (est)
-
保留指数:1275
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:10
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
储存条件:2-8°C
SDS
反应信息
-
作为反应物:参考文献:名称:Absolute Configuration and Conformation of 1,3-Dioxanes from Cider摘要:In extracts obtained by Liquid-liquid extraction from French cider (2S,4R)-and (2R,4R)-2-methyl-4-pentyl-1,3-dioxane, 1a and 1b as well as (2S,4R)-and (2R,4R)-2-methyl-4-(2'(Z)-pentenyl)-1,3-dioxane, 2a and 2b, were identified by capillary gas chromatography (HRGC) and capillary gas chromatography-mass spectrometry (HRGC-MS). Absolute configuration and conformation of the 1,3-dioxanes was determined by nuclear magnetic resonance (NMR) spectrometry techniques [C-13, H-1, nuclear Overhauser enhancement (NOE), and WH homonuclear decoupling], multidimensional gas chromatography (MDGC), and by comparison with synthesized reference compounds. A nonenzymatic formation of la and Ib and 2a and 2b during fermentation of apple juice was proposed leading to 22, 8, 2, and < 1 mg/L of la, 2a, 1b, and 2b, respectively in cider.DOI:10.1021/jf970162n
-
作为产物:参考文献:名称:来自 Carica pubescens 果实的脂肪族 β-d-葡萄糖苷摘要:摘要 采用XAD液相色谱法从毛竹果肉中分离得到3-O-β-d-吡喃葡萄糖基丁酸乙酯、3-O-β-d-吡喃葡萄糖基丁酸丁酯和3-氧辛基1-O-β-d-吡喃葡萄糖苷。通过将 HRGC 和 HRGC 质谱数据与合成参考化合物的数据进行比较,在全乙酰化后进行鉴定。通过多维气相色谱,将极性非手性柱 (DB-Wax) 与手性主柱 (heptakis-2,6-di-O -甲基-3-O-戊基-β-环糊精/OV 1701 和 2, 3-二-O-乙酰基-6-O-叔丁基-二甲基甲硅烷基-β-环糊精/OV 1701)。合成的保留时间比较,具有酶促释放苷元的光学富集参考化合物显示(S)-3-羟基丁酸酯的对映体过量,即乙酯和丁酯分别为96%和24%。辛烷-1,3-二醇表现出 ( R )-90% 的对映体过量。© 1997 Elsevier Science Ltd. 版权所有DOI:10.1016/s0031-9422(97)00196-9
文献信息
-
Extracts of Isochrysis sp.申请人:Herrmann Martina公开号:US20100080761A1公开(公告)日:2010-04-01The present invention relates to extracts of Isochrysis sp., preferably Tahitian Isochrysis, its cosmetic, dermatological and/or therapeutic uses and compositions and cosmetic, dermatological or therapeutic products comprising such an extract of Isochrysis sp., preferably Tahitian Isochrysis.
-
Selective Sulfonylation of Arenes and Benzoylation of Alcohols Using Lithium Perchlorate as a Catalyst Under Neutral Conditions作者:B. P. Bandgar、V. T. Kamble、V. S. Sadavarte、L. S. UppallaDOI:10.1055/s-2002-25345日期:——Sulfonylation of aromatics with p-toluenesulfonyl chloride and benzoylation of alcohols with benzoyl chloride using lithium perchlorate as a catalyst is described. The remarkable selectivity under neutral conditions is an attractive feature of this method
-
A Single Point Mutation Alters the Transglycosylation/Hydrolysis Partition, Significantly Enhancing the Synthetic Capability of an <i>endo</i>-Glycoceramidase作者:Julien Durand、Xevi Biarnés、Laurie Watterlot、Cyrielle Bonzom、Vinciane Borsenberger、Antoni Planas、Sophie Bozonnet、Michael J. O’Donohue、Régis FauréDOI:10.1021/acscatal.6b02159日期:2016.12.2The mutation of D311 to tyrosine in endo-glycoceramidase II from Rhodococcus sp. and the use of a poorly recognized substrate, 2-chloro-4-nitrophenyl β-cellobioside, have provided appropriate conditions for the efficient synthesis of alkyl β-cellobioside derivatives. The mutant D311Y was characterized by a lowered KM value for the hydrolysis of 2-chloro-4-nitrophenyl β-cellobioside and increased transglycosylation
-
EXTRACTS OF TETRASELMIS SP.申请人:Pertile Paolo公开号:US20100143267A1公开(公告)日:2010-06-10The present invention relates to extracts of Tetraselmis sp., preferably Tetraselmis suecica , its cosmetic, dermatological and/or therapeutic uses and compositions and cosmetic, dermatological or therapeutic products comprising such an extract of Tetraselmis sp., preferably Tetraselmis suecica.
-
Novel cytarabine monophosphate prodrugs申请人:——公开号:US20040092476A1公开(公告)日:2004-05-13Compounds of Formula I, their preparation and uses are described: 1 wherein: M and V are cis to one another and MH is cytarabine; the 5′ oxygen of said cytarabine is attached to the phosphorus; V is 4-pyridyl; and pharmaceutically acceptable prodrugs and salts thereof.
表征谱图
-
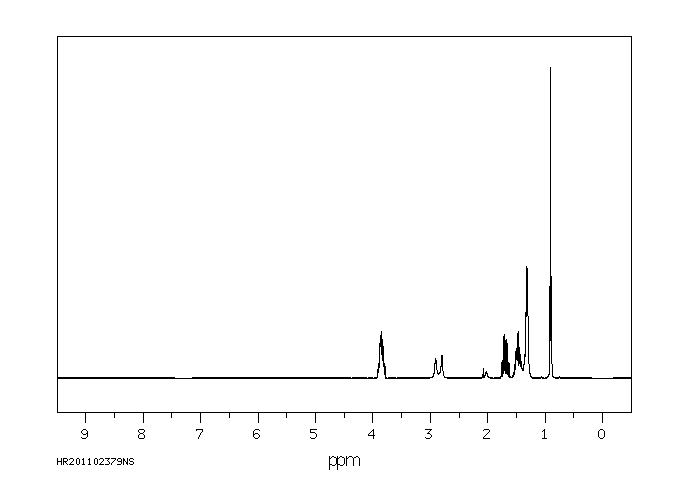
氢谱1HNMR
-
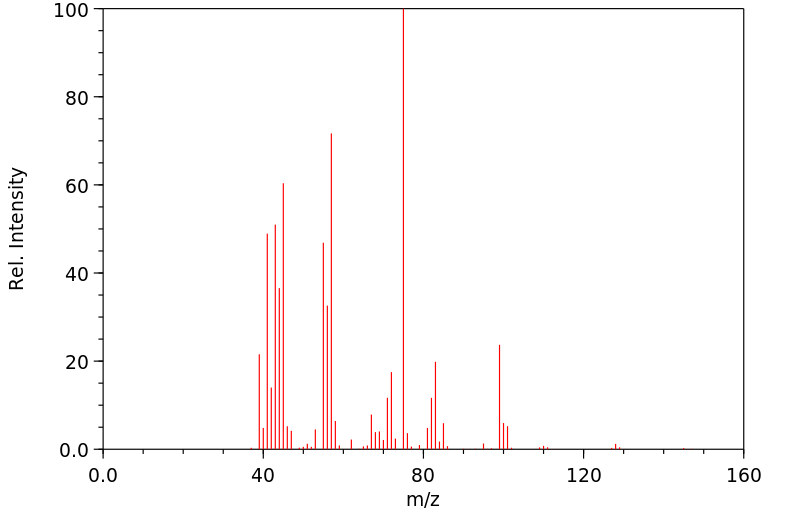
质谱MS
-
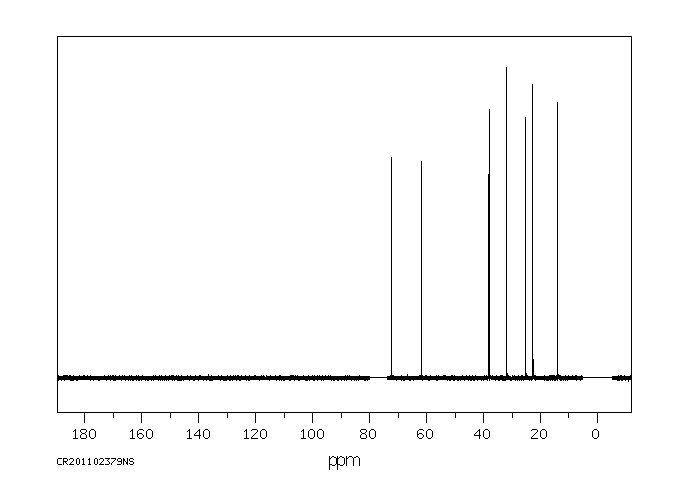
碳谱13CNMR
-
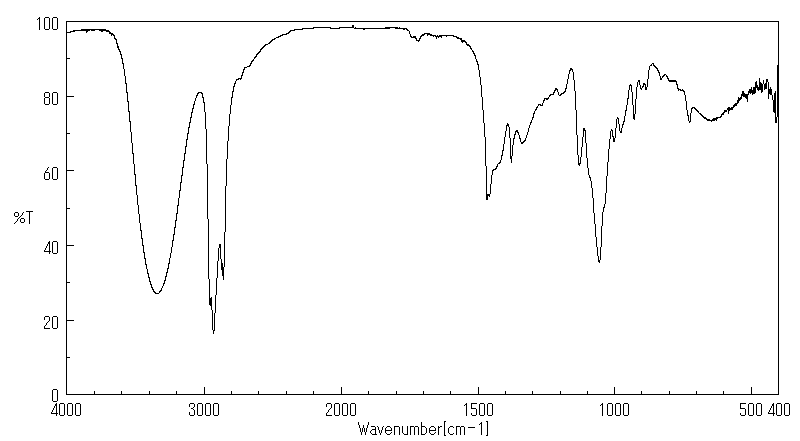
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯