1-氨基-2-甲基吡啶碘化物 | 7583-90-6
中文名称
1-氨基-2-甲基吡啶碘化物
中文别名
——
英文名称
1-amino-2-methylpyridinium iodide
英文别名
2-methylpyridin-1-ium-1-amine;iodide
CAS
7583-90-6
化学式
C6H9N2*I
mdl
——
分子量
236.055
InChiKey
XMFXSCPQGJZSLU-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:148-149℃
计算性质
-
辛醇/水分配系数(LogP):-3.0
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:29.9
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2933399090
-
储存条件:应储存在室温、密封和干燥的环境中。
SDS
1-Amino-2-methylpyridinium Iodide Revision number: 5.3
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1-Amino-2-methylpyridinium Iodide
Revision number: 5.3
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1-Amino-2-methylpyridinium Iodide
Percent: >98.0%(HPLC)(T)
CAS Number: 7583-90-6
Chemical Formula: C6H9IN2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
1-Amino-2-methylpyridinium Iodide
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
1-Amino-2-methylpyridinium Iodide
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Colour: White - Slightly pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:152°C (dec.)
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen Iodide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
1-Amino-2-methylpyridinium Iodide
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1-Amino-2-methylpyridinium Iodide
Revision number: 5.3
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1-Amino-2-methylpyridinium Iodide
Percent: >98.0%(HPLC)(T)
CAS Number: 7583-90-6
Chemical Formula: C6H9IN2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
1-Amino-2-methylpyridinium Iodide
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
1-Amino-2-methylpyridinium Iodide
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Colour: White - Slightly pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:152°C (dec.)
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen Iodide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
1-Amino-2-methylpyridinium Iodide
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Kakehi, Akikazu; Ito, Suketaka; Nagata, Kenji, Chemical and pharmaceutical bulletin, 1987, vol. 35, # 1, p. 156 - 169摘要:DOI:
-
作为产物:描述:参考文献:名称:非咪唑杂环组胺H3受体拮抗剂。摘要:围绕咪唑并吡啶组胺H(3)拮抗剂前导的SAR的继续探索导致了几个相关系列的杂环组胺H(3)拮抗剂的发现。现在描述吲哚嗪,吲哚和吡唑并吡啶基化合物的合成和SAR。DOI:10.1016/s0960-894x(03)00299-3
文献信息
-
Composition for the dyeing of keratin fibers comprising at least one 3-amino-pyrazolopyridine derivatives申请人:Fadli Aziz公开号:US20070143935A1公开(公告)日:2007-06-28Disclosed herein is a composition for the dyeing of keratin fibers, for example, human keratin fibers such as the hair, comprising at least one 3-amino pyrazolo-[1,5-a]-pyridine derivative or one of its addition salts and a method employing this derivative. The composition of the present disclosure makes it possible to obtain coloration in various shades that is powerful, chromatic, and aesthetic, with low selectivity and with good resistance to various aggressive factors to which the hair may be subjected, such as shampoos, the light, sweat and permanent shaping.
-
[3+2] Cycloaddition of N-Aminopyridines and Perfluoroalkynylphosphonates: Facile Synthesis of Perfluoroalkylated Pyrazolo[1,5-a]pyridines Containing a Phosphonate Moiety作者:Hui Zhang、Weiguo Cao、Qi Huang、Dong He、Jing Han、Jie Chen、Weimin He、Hongmei Deng、Min ShaoDOI:10.1055/s-0037-1610443日期:2018.93-Zwitterions generated from N-aminopyridines in the presence of base are trapped by perfluoroalkynylphosphonates to yield a variety of perfluoroalkylated pyrazolo[1,5-a]pyridine derivatives bearing a phosphonate group. The salient features of these [3+2] cycloadditions include operational simplicity, good tolerance of functional groups, and good to excellent yields at room temperature. 1,3-Zwitterions generated
-
Synthesis Using Allylidenedihydropyridines. VIII. Facile Preparation of 2-Alkylthio-3-vinylpyrazolo[1,5-a]pyridines作者:Akikazu Kakehi、Suketaka Ito、Kozo WatanabeDOI:10.1246/bcsj.53.1775日期:1980.6Reactions of 1-[bis(alkylthio)methyleneamino]-2-methylpyridinium iodides with activated ethoxymethylene compounds in the presence of alkali gave the corresponding 1-[bis(alkylthio)methyleneamino]-2-allylidene-1,2-dihydropyridines in considerable yields, and their thermolyses in benzene afforded 2-alkylthio-3-vinylpyrazolo[1,5-a]pyridine derivatives.
-
Preparation of New Nitrogen-Bridged Heterocycles. 42. Synthesis and the Reaction of Pyridinium<i>N</i>-Ylides Using Bifunctional Ethyl Thiocyanatoacetates作者:Akikazu Kakehi、Suketaka Ito、Yasunobu HashimotoDOI:10.1246/bcsj.69.1769日期:1996.6cycloadditions of some pyridinium (unsymmetrically substituted cyanomethylide)s with dimethyl acetylenedicarboxylate (DMAD) in various solvents afforded only dimethyl 3-cyanoindolizine-1,2-dicarboxylate, except a few examples. On the other hand, the treatment of pyridinium (thiocyanatoaceto)- or (2-thiocyanatopropiono)amidates with a strong base, such as potassium t-butoxide, gave new bicyclic mesoionic compounds各种吡啶鎓(单取代的甲基化物)在硫氰酸乙酯或 2-硫氰酸根合丙酸乙酯中顺利地攻击氰基,以低到中等的产率得到相应的吡啶鎓(取代的氰基甲基化物),而吡啶鎓(未取代的酰胺化物)与在相同的试剂中酯羰基以可观的产率得到吡啶鎓(硫氰酸根合乙酰基)-或(2-硫氰酸根合丙基)酰胺。除了几个例子外,一些吡啶鎓(不对称取代的氰基甲基化物)与乙炔二甲酸二甲酯 (DMAD) 在各种溶剂中的 1,3-偶极环加成仅得到 3-氰基吲哚腙-1,2-二甲酸二甲酯。另一方面,用强碱(如叔丁醇钾)处理吡啶鎓(硫氰基乙酰基)-或(2-硫氰基丙酸基)酰胺,得到了新的双环介离子化合物 N-[2-(1,3,4-噻二唑并[3,2-a]吡啶并)]乙酰胺衍生物,收率适中。N-[1-(2-thiocyanatopyridinio)]aceta的中间体...
-
Preparation of New Nitrogen-bridged Heterocycles. 11. A New Synthetic Method of Pyrazolo[1,5-<i>a</i>]pyridines from the Alkaline Treatment of 1-[(Acylmethylthio)methyleneamino]pyridinium Salts作者:Akikazu Kakehi、Suketaka Ito、Masayoshi Ito、Toshiaki Yotsuya、Kenji NagataDOI:10.1246/bcsj.58.1432日期:1985.5Alkaline treatment of 1-[(acylmethylthio)methyleneamino]pyridinium halides gave unexpectedly 3-acyl- or 3-(acylthio)pyrazolo[1,5-a]pyridine derivatives in 35–87% yields. Some 4,4a-dihydropyrido[1,2-d][1,3,4]thiadiazine intermediates involved in these reactions could be detected by their nmr follows and three related 4a,8-dimethyl derivatives were isolated. Substituent effect and possible mechanisms
表征谱图
-
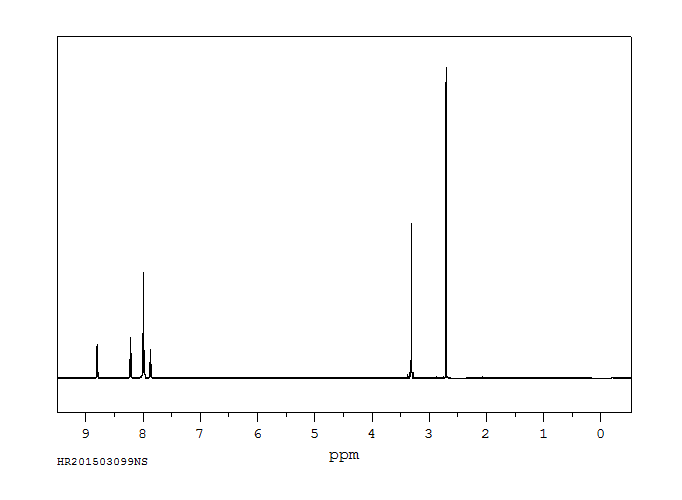
氢谱1HNMR
-
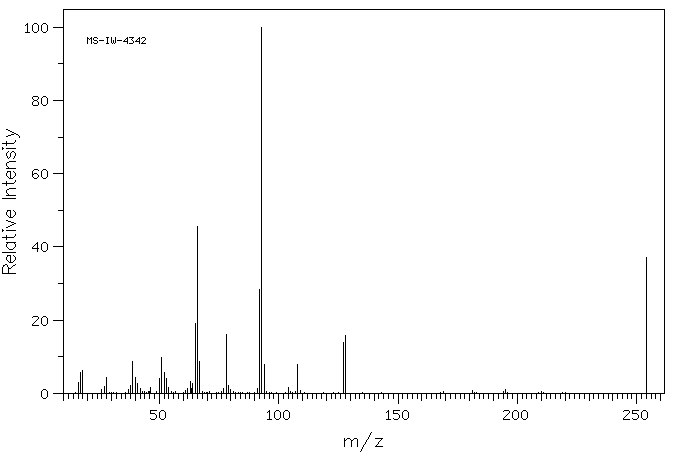
质谱MS
-
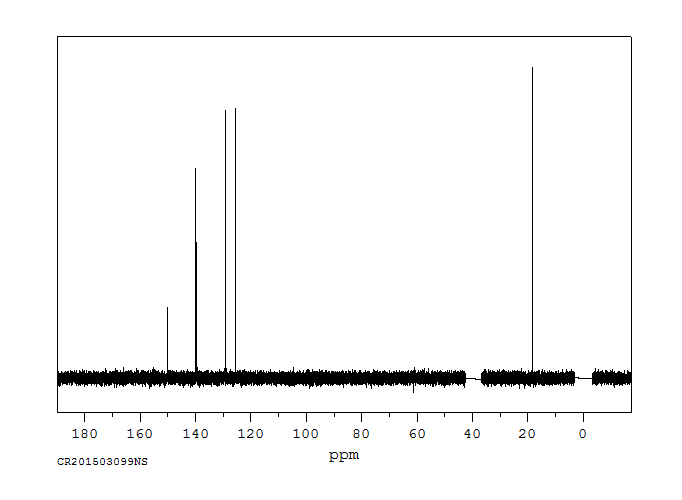
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-