2-氨基-2,4,6-环庚三烯-1-酮 | 6264-93-3
中文名称
2-氨基-2,4,6-环庚三烯-1-酮
中文别名
(2E,4Z,6Z)-2-氨基环庚-2,4,6-三烯酮
英文名称
2-aminotropone
英文别名
2-Amino-tropon;2-amino-2,4,6-cycloheptatrien-1-one;2-aminocyclohepta-2,4,6-trien-1-one
CAS
6264-93-3
化学式
C7H7NO
mdl
MFCD01734302
分子量
121.139
InChiKey
PIOPKWFBKZTUMS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:106.5°C
-
沸点:225.84°C (rough estimate)
-
密度:1.1344 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:43.1
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险性防范说明:P264,P280,P302+P352,P337+P313,P305+P351+P338,P362+P364,P332+P313
-
危险性描述:H315,H319
-
储存条件:应存放在2-8°C的环境中,并避免光照,建议采用干燥密封的方式保存。
SDS
制备方法与用途
反应信息
-
作为反应物:描述:2-氨基-2,4,6-环庚三烯-1-酮 在 盐酸 、 potassium tert-butylate 作用下, 以 二氯甲烷 为溶剂, 反应 8.0h, 生成 10-硫杂-8-氮杂双环[5.3.0]癸-1,3,5,7-四烯-9-酮参考文献:名称:Convenient Preparation of Heteroazulenes by the Reaction of 2-Aminotropone with Heterocumulenes in the Presence of a Base摘要:DOI:10.3987/com-01-9322
-
作为产物:描述:参考文献:名称:1-氮杂氮烯和氮杂烯组成的线性π共轭分子的合成与性质摘要:使用Sonogashira-Hagihara交叉偶联反应,然后用浓磷酸脱羧,合成了两种化合物6-(1-(氮杂氮烯-2-基)乙炔基氮杂烯(8)和6-(2-氮杂烯基)乙炔基氮杂烯(10)。化合物8和10的特征在于1 H和13 C核磁共振(NMR)光谱,质谱,紫外可见(UV-Vis)光谱,循环伏安法和密度泛函理论(DFT)计算。根据该结果,证实两种化合物在其整个分子结构中均具有π共轭。化合物8和10的酸响应度使用UV-Vis和1 H NMR光谱进行了评估。发现化合物8对三氟乙酸高度敏感,其1-氮杂氮烯基部分充当碱。当与过量的三氟乙酸混合时,化合物10产生z阳离子。DOI:10.1016/j.tet.2019.130658
-
作为试剂:描述:3-苯基丙-2-烯-1-醇 在 2-氨基-2,4,6-环庚三烯-1-酮 、 potassium tert-butylate 、 三苯基二氯化铋 作用下, 以 二氯甲烷 为溶剂, 反应 5.0h, 以85%的产率得到反式肉桂醛参考文献:名称:A Convenient in situ Preparation of Triphenylbismuthane (Tropon-2-yl)imide: Reaction with Heterocumulenes and Activated Alcohols摘要:The first in situ preparation of triphenylbismuthane (tropon-2-yl)imide has been accomplished by the reaction of triphenylbismuth dichloride with 2-aminotropone. The imide is not isolated due to its moisture sensitivity, while it undergoes aza-Wittig type reaction with heterocumulenes leading to cyclohepta-annulated heterocycles in situ, and reacts also as an oxidizing agent of activated alcohols to give the corresponding carbonyl compounds under mild conditions.DOI:10.3987/com-01-9156
文献信息
-
Synthesis, structure, and reactivity of (tropon-2-ylimino)arsorane and in situ generation of its stiborane and bismuthorane analogues: reactions with heterocumulenes and an activated acetylene giving heteroazulenes作者:Makoto Nitta、Yuhki Mitsumoto、Hiroyuki YamamotoDOI:10.1039/b103098c日期:——(Tropon-2-ylimino)pnictoranes of the general structure RNMPh3 (R = tropon-2-yl; M = As, Sb, and Bi) 4–6 have been prepared for the first time by the reaction of 2-aminotropone with Ph3MX2 (M = As, Sb, and Bi) in the presence of a base. The arsorane derivative (M = As) 4 is isolated as a stable crystalline compound, while the stiborane (M = Sb) and the bismuthorane (M = Bi) derivatives 5 and 6 are not isolated and are(Tropon -2-基亚氨基)的一般结构的pnictoranes RN MPh的3(R = tropon -2-基; M =砷,锑和Bi)4-6已经由2- aminotropone的反应首次制备在碱的存在下用Ph 3 MX 2(M = As,Sb和Bi)制成。这rs衍生物(M = As)4作为稳定的结晶化合物被分离出来,而七硼烷(M = Sb)和双甲硼烷(M = Bi)衍生物5和6未被分离,由于它们对水分的敏感性而被原位制备。X射线晶体分析表明,化合物4在固态下表现出两种不同的构型,并且As–O键距(2.33Å)低于范德华半径的总和(3.37Å),因此,是之间的明显的键相互作用rs和氧原子。为了构建一系列环庚环杂环并更好地理解一系列亚吡咯烷烷,允许化合物4-6与二甲基碳杂杂环烯反应,异硫氰酸苯酯, 异氰酸苯酯, 和 二苯基碳二亚胺,在aza-Wittig /电环化或正式的[8 + 2]型
-
Oxidizing Ability of a Series of (Tropon-2-ylimino)pnictoranes (Pnictogen = P, As, Sb, and Bi) toward Some Alcohols作者:Yuhki Mitsumoto、Makoto NittaDOI:10.1246/bcsj.76.1029日期:2003.5In order to gain a better understanding of the oxidizing ability of a series of (tropon-2-ylimino)pnictoranes of the general structure Ph3M=NR (R = tropon-2-yl; M = P, As, Sb, and Bi), reactions were run with some alcohols such as benzopinacol (1,1,2,2-tetraphenyl-1,2-ethanediol), benzoin, cinnamyl alcohol, 1-phenylethanol, a mixture of cis- and trans-4-t-butylcyclohexanol, 2-phenylethanol, and 1-phenyl-1,3-propandiol. Iminophosphorane oxidized only benzopinacol to give benzophenone, while both arsorane and stiborane oxidized benzopinacol and benzoin to give benzophenone and benzil, respectively. On the other hand, iminobismuthorane has appreciable oxidizing ability, and reacted with the alcohols mentioned above, except the primary alcohol, to give the corresponding carbonyl compounds. Iminobismuthorane reacted with 1-phenyl-1,3-propandiol selectively to oxidize benzyl alcohol moiety, but not a primary alcohol moiety, to give 3-hydroxy-1-phenyl-1-propanone. Thus, the oxidizing ability of a series of (tropon-2-ylimino)pnictoranes is demonstrated to be in the order of iminophosphorane < iminoarsorane < iminostiborane < iminobismuthorane: the dipolar (degree of contribution of the Ph3M+–−NR canonical structure) and electrophilic character of pnictogen elements of a series of iminopnictoranes appear to increase their oxidizing ability when the pnictogen stands lower in the periodic table.为了更好地理解一系列具有Ph3M=NR通式结构(R=2-亚芴基;M=P、As、Sb和Bi)的(2-亚芴基亚氨)膦烷类物质的氧化能力,我们进行了一些与醇类反应的实验,比如苯并匹考啉(1,1,2,2-四苯基-1,2-乙二醇),苯偶姻,肉桂醇,1-苯乙醇,顺-和反-4-叔丁基环己醇的混合物,2-苯乙醇,以及1-苯基-1,3-丙二醇。亚氨膦烷只将苯并匹考啉氧化生成了苯酞酮,而亚砷烷和锑烷都分别把苯并匹考啉和苯偶姻氧化生成了苯酞酮和苯偶酰。另一方面,亚铋锑烷具有显著的氧化能力,并且引发了上述提到的除一元醇以外的所有醇类,生成了相应的羰基化合物。亚铋锑烷与1-苯基-1,3-丙二醇选择性地反应,仅将苄醇基团氧化为3-羟基-1-苯基-1-丙酮,并未氧化一元醇基团。因此,一系列(2-亚芴基亚氨)膦烷类物质的氧化能力可证明是按照亚氨膦烷<亚砷烷<锑烷<铋锑烷的顺序排列:当膦元素在周期表中的位置越低,其偶极矩(Ph3M+–−NR经典结构贡献程度)和亲电特性会使得一系列亚氨膦烷的氧化能力增强。
-
Studies on Seven-membered Ring Compounds. XX. Reactions of Troponeimine Derivatives. (2)作者:Hideo Nakao、Nobuo Soma、Genshun SunagawaDOI:10.1248/cpb.13.828日期:——The reaction of 2-methoxytroponeimine (I) and its N-methyl derivative (II) with active methylene compounds were carried out. The reaction of I with malononitrile, ethyl cyanoacetate or cyanoacetamide in the presence of sodium ethoxide afforded rearrangemet products, that is 3-phenylacrylic acid derivatives. The same reaction in the absence of sodium ethoxide gave mainly 2-imino-1, 2-dihydrocyclohepta[b]pyrrole derivatives. However, the reaction of I with diethyl malonate afforded ethyl 2-oxo-8-methoxy-1, 2-dihydrocyclohepta[b]pyrrole-3-carboxylate. II showed some different reactivity from I. Namely, the reaction of II with active methylene compounds in the presence of sodium ethoxide afforded 2-imino-1, 2-dihydrocyclohepta[b]pyrrole derivatives besides rearrangement products.
-
A Convenient Synthesis of 5-Aryltropolones via Novel Benzidine Type Rearrangement of 2-(2-Arylhydrazino)tropones作者:Tetsuo Nozoe、Kahei Takase、Hiroaki Saito、Hiroshi Yamamoto、Kimiaki ImafukuDOI:10.1246/cl.1986.1577日期:1986.9.5Treatment of a wide variety of 2-(2-arylhydrazino)tropones with ethanolic acid at 50–80 °C readily gave the benzidine type rearrangement products, i.e. 2-amino-5-(4-aminoaryl)tropones, which in turn were conveniently led to the corresponding 5-aryltropolones that can be utilized for synthesizing B-ring-open analogues of colchicine.
-
Troponyl-oxamic acid derivatives申请人:Ayerst, McKenna & Harrison Limited公开号:US04125625A1公开(公告)日:1978-11-14Tropone derivatives characterized by having a derivative of oxamic acid at positions 2 and or 5 are disclosed. In addition, the tropone nucleus can be optionally further substituted. The foregoing compounds are useful for preventing or treating allergic conditions in a mammal. Methods for the preparation and use of said compounds are disclosed.
表征谱图
-
氢谱1HNMR
-
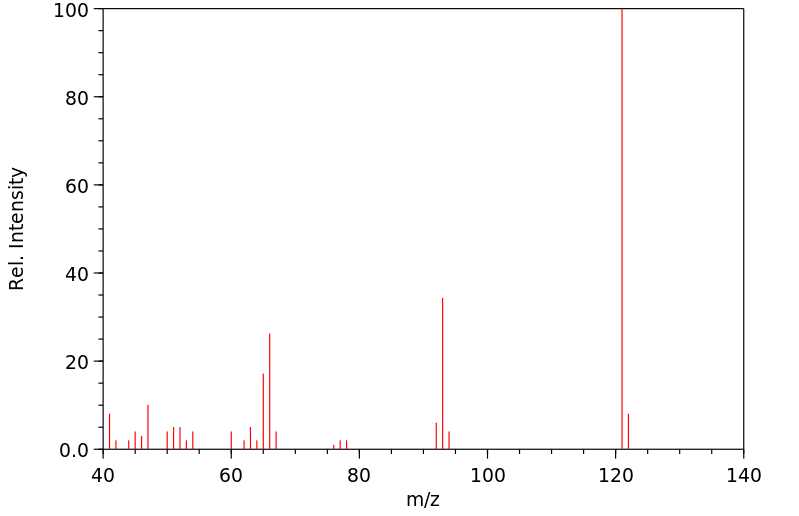
质谱MS
-
碳谱13CNMR
-
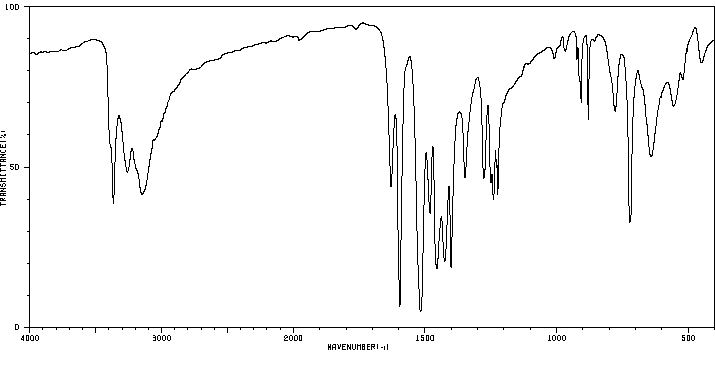
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
脱羰秋水仙碱
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
硫代秋水仙碱
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
环庚三烯酮
环庚三烯酚酮
氨甲酸,(1-乙基戊基)-,甲基酯(9CI)
桧木醇
异秋水仙胺
尼楚酮
对二硫辛酸
双环[4.4.1]十一碳-1(10),2,4,6,8-五烯-11-酮
双环[4.1.0]庚-1,3,5-三烯-7-酮
去乙酰氨基秋水仙碱
原秋水仙碱
十四烷酸,4-(十八烷氧基)-7-羰基-1,3,5-环庚三烯-1-基酯
乙基[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]氨基甲酸酯
三甲基秋水仙素酸
三甲基秋水仙素酸
三(2-羟基-2,4,6-环庚三烯-1-酮)-铟
α-(异丙基)-γ,γ-二甲基环己丙醇
beta-斧松素
[(7S)-7-乙酰氨基-1,3-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-2-基]2-氯乙酸酯
[(7S)-7-乙酰氨基-1,2-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-3-基]2-氯乙酸酯
N-(2-巯基乙基)秋水仙胺
N-脱乙酰基3-去甲基硫代秋水仙碱
N-脱乙酰基,1,2,3,10-脱甲基秋水仙碱
N-甲酰脱乙酰秋水仙碱
N-甲酰基秋水仙胺
N-甲基-秋水仙碱
N-三氟乙酰基-N-甲基-去乙酰基秋水仙碱
N-[(S)-5,6,7,9-四氢-1,2,3,10-四甲氧基-9-氧代苯并[a]庚搭烯-7-基]-2,2,2-三氟乙酰胺
N-[(7S)-4-(羟基甲基)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-10-(丁基氨基)-5,6,7,9-四氢-1,2,3-三甲氧基-9-氧代苯并[a]庚搭烯-7-基]-乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲硫基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲基氨基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]丙酰胺
N-[(7R)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-(乙氧基乙酰基)去乙酰基硫代秋水仙碱
N-(5,6,7,9-四氢-1,2,3-三甲氧基-10-甲硫基-9-氧代苯并[a]庚搭烯-7-基)氨基甲酸乙酯
N-(4-甲酰基-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(10-二甲基氨基-1,2,3-三甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,9-四甲氧基-10-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基)乙酰胺
9H-三苯并[A,C,E][7]环轮烯-9-酮
8H-环庚三烯并[c]异噻唑-8-酮,3-甲基-