1-(1-adamantyl)-3,4-dimethylbenzene | 62133-11-3
中文名称
——
中文别名
——
英文名称
1-(1-adamantyl)-3,4-dimethylbenzene
英文别名
4-(1-Adamantyl)-1,2-dimethylbenzene;1-(3,4-dimethylphenyl)adamantane;4-(1-Adamantyl)-o-xylene;1-(3,4-xylyl)adamantane
CAS
62133-11-3
化学式
C18H24
mdl
——
分子量
240.389
InChiKey
KXXQPTCMSHPHIX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:110-112 °C(Solv: ethanol (64-17-5))
-
沸点:346.7±22.0 °C(Predicted)
-
密度:1.040±0.06 g/cm3(Predicted)
-
保留指数:1978
计算性质
-
辛醇/水分配系数(LogP):6.1
-
重原子数:18
-
可旋转键数:1
-
环数:5.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
反应信息
-
作为反应物:描述:1-(1-adamantyl)-3,4-dimethylbenzene 在 lithium perchlorate 、 sodium carbonate 、 三氟乙酸 、 叔丁醇 作用下, 以 二氯甲烷 为溶剂, 以47%的产率得到3-(3,4-二甲基苯基)-1-金刚烷醇参考文献:名称:Kovalev, V. V.; Knyazeva, I. V.; Shokova, E. A., Journal of Organic Chemistry USSR (English Translation), 1986, vol. 22, p. 692 - 696摘要:DOI:
-
作为产物:描述:2-(3,4-dimethylphenyl)adamantane 在 三氯化铝 作用下, 以 xylene 为溶剂, 生成 1-(1-adamantyl)-3,4-dimethylbenzene参考文献:名称:Pimerzin; Sarkisova, Petroleum Chemistry, 2003, vol. 43, # 2, p. 94 - 102摘要:DOI:
文献信息
-
Synergistic Brønsted/Lewis acid catalyzed aromatic alkylation with unactivated tertiary alcohols or di-<i>tert</i>-butylperoxide to synthesize quaternary carbon centers作者:Aaron Pan、Maja Chojnacka、Robert Crowley、Lucas Göttemann、Brandon E. Haines、Kevin G. M. KouDOI:10.1039/d1sc06422c日期:——Dual Brønsted/Lewis acid catalysis involving environmentally benign, readily accessible protic acid and iron promotes site-selective tert-butylation of electron-rich arenes using di-tert-butylperoxide. This transformation inspired the development of a synergistic Brønsted/Lewis acid catalyzed aromatic alkylation that fills a gap in the Friedel–Crafts reaction literature by employing unactivated tertiary
-
Direct Clay-Catalyzed<i>Friedel</i>-<i>Crafts</i>Arylation and Chlorination of the Hydrocarbon Adamantane作者:Stéphane Chalais、André Cornélis、André Gerstmans、Wacław Kołodziejski、Pierre Laszlo、Arthur Mathy、Pierre MétraDOI:10.1002/hlca.19850680516日期:1985.8.14Multiple chlorinations and arylations at the tertiary positions of adamantane are promoted by FeCl3-doped K10 montmorillonite in CCl4 or in aromatic solvents. The process, remarkably easy to implement, can be tailored to selective formation of monosubstituted 1-adamantyl derivatives or 1,3-disubstituted adamantanes. The process achieves alkylation at the meta- and para-positions of toluene leading
-
Flow <scp>Friedel–Crafts</scp> alkylation of <scp>1‐adamantanol</scp> with arenes using <scp>HO‐SAS</scp> as an immobilized acid catalyst作者:Takayoshi Kasakado、Mamoru Hyodo、Akihiro Furuta、Aina Kamardine、Ilhyong Ryu、Takahide FukuyamaDOI:10.1002/jccs.202000518日期:2020.12In this communication flow Friedel–Crafts alkylation was studied using hydroxy‐substituted sulfonic acid‐functionalized silica as a catalyst and 1‐adamantanol as a model substrate. The reaction of 1‐adamantanol (1a) with toluene (2a) proceeded well with 5 min of residence time at 120°C to give good yield of 1‐tolyladamantane (3a) as a 1:9 mixture of meta and para isomers. When the flow synthesis was
-
Braese, Stefan; Waegell, Bernard; Meijere, Armin de, Synthesis, 1998, # 2, p. 148 - 152作者:Braese, Stefan、Waegell, Bernard、Meijere, Armin deDOI:——日期:——
-
Alkylation of aromatic compounds with adamantan-1-ol作者:A. V. Stepakov、A. P. Molchanov、R. R. KostikovDOI:10.1134/s1070428007040082日期:2007.4Reactions of substituted benzenes, naphthalenes, and 2- and 3-phenyl-1-benzofurans with adamantan-1-ol in trifluoroacetic acid lead to the formation of the corresponding mono-and diadamantylsubstituted aromatic compounds.
表征谱图
-
氢谱1HNMR
-
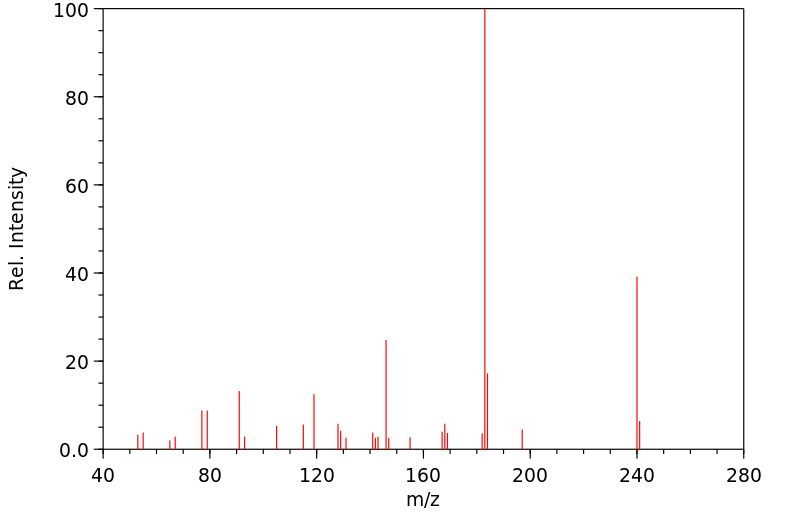
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫