苄基三正丙基氯化铵 | 5197-87-5
中文名称
苄基三正丙基氯化铵
中文别名
苄基三丙基氯化铵;氯化铵三丙基苄基铵;苄基三-正-丙基氯化铵
英文名称
benzyltripropylammonium chloride
英文别名
benzyl(tripropyl)azanium;chloride
CAS
5197-87-5
化学式
C16H28N*Cl
mdl
——
分子量
269.858
InChiKey
YTRIOKYQEVFKGU-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:~180 °C (dec.)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):1.24
-
重原子数:18
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.62
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2923900090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将药品存放在避光、阴凉干燥的地方,并密封保存。
SDS
反应信息
-
作为反应物:描述:(2S,5R)-6-hydroxy-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide 、 苄基三正丙基氯化铵 在 三甲基铵三氧化硫共聚物 、 三乙胺 作用下, 以 乙醇 、 水 为溶剂, 以67.8%的产率得到参考文献:名称:一种阿维巴坦中间体的合成方法摘要:本发明提供一种阿维巴坦中间体的合成方法,所述方法包括以下步骤:(1)(2S,5R)‑6‑羟基‑7‑氧代‑1,6‑二氮杂二环[3.2.1]辛烷‑2‑甲酰胺、三乙胺、三氧化硫三甲胺络合物三者进行反应;(2)洗涤反应液,加入氯化季铵盐在水中的溶液,继续反应;(3)萃取产物并浓缩,打浆,过滤、洗涤并干燥;本发明操作简便、节约工时与能耗,得到的季铵盐中间体纯度较高,杂质的含量由现有技术的5.3%下降到0.14%以下,更适合工艺生产放大。公开号:CN106831772B
-
作为产物:参考文献:名称:Makosza,M.; Serafinowa,B., Roczniki Chemii, 1965, vol. 39, p. 1223 - 1231摘要:DOI:
-
作为试剂:描述:5,5-二甲基-3-异噁唑烷酮 、 溴甲苯 在 sodium hydroxide 、 苄基三正丙基氯化铵 作用下, 以 二氯甲烷 为溶剂, 反应 15.0h, 以34.3%的产率得到3-benzyloxy-5,5-dimethyl-4,5-dihydro-isoxazole参考文献:名称:Dehmlow, Eckehard Volker; Bollhoefer, Joerg; Thye, Gorden, Journal of Chemical Research - Part S, 2001, # 3, p. 113 - 115摘要:DOI:
文献信息
-
Process for producing acrylic acid derivative申请人:Nippon Soda Co. Ltd公开号:US20040152894A1公开(公告)日:2004-08-05Processes for producing a compound represented by the formula (1), which includes an acrylic acid derivative and is useful as an agricultural chemical or medicine. One of the processes comprises the step of formulating a compound (3) and converting the OH of the resultant compound (2) into OR″. The first step comprises reacting a formic or orthoformic ester in the presence of a Lewis acid and a base. The second step comprises reacting the compound with R″OH or with R″OH and CH(OR″) 3 under acidic conditions or using a phase-transfer catalyst in a two-phase system and regulating the base and the concentration thereof to stereoselectively synthesize the target compound. In another, process, the compound is efficiently produced without isolating the compound. The compound can also be produced without the compound (2). 1
-
[EN] SYNTHETIC INTERMEDIATE OF OXAZOLE COMPOUND AND METHOD FOR PRODUCING THE SAME<br/>[FR] INTERMÉDIAIRE SYNTHÉTIQUE D'UN COMPOSÉ OXAZOLE ET PROCÉDÉ DE PRODUCTION ASSOCIÉ申请人:OTSUKA PHARMA CO LTD公开号:WO2011093529A1公开(公告)日:2011-08-04An object of the present invention is to provide a method for producing an oxazole compound in a high yield. The object can be achieved by a compound represented by Formula (11): wherein R1 is a hydrogen atom or lower-alkyl group; R2 is a 1-piperidyl group substituted at the 4-position with a substituent selected from (A1a) a phenoxy group substituted on the phenyl moiety with one or more halogen-substituted lower-alkoxy groups, (A1b) a phenoxy-substituted lower-alkyl group substituted on the phenyl moiety with one or more halogen-substituted lower-alkyl groups, (A1c) a phenyl-substituted lower-alkoxy lower-alkyl group substituted on the phenyl moiety with halogen, (A1d) a phenyl-substituted lower-alkyl group substituted on the phenyl moiety with one or more halogen-substituted lower-alkoxy groups, (A1e) an amino group substituted with a phenyl group substituted with one or more halogen-substituted lower-alkoxy groups, and a lower-alkyl group, and (A1f) a phenyl-substituted lower-alkoxy group substituted on the phenyl moiety with one or more halogen-substituted lower-alkoxy groups; n is an integer from 1 to 6; and X3 is an organic sulfonyloxy group.本发明的目的是提供一种高产率生产噁唑化合物的方法。该目的可以通过由式(11)表示的化合物实现:其中R1是氢原子或较低的烷基基团;R2是在4-位被取代的1-哌啶基团,所述取代基选自(A1a)苯氧基在苯基上取代一个或多个卤素取代的较低烷氧基团,(A1b)苯氧基取代的较低烷基基团在苯基上取代一个或多个卤素取代的较低烷基基团,(A1c)苯基取代的较低烷氧基较低烷基基团在苯基上取代卤素,(A1d)苯基取代的较低烷基基团在苯基上取代一个或多个卤素取代的较低烷氧基团,(A1e)氨基取代的苯基取代一个或多个卤素取代的较低烷氧基团和较低烷基基团,以及(A1f)苯基取代的较低烷氧基团在苯基上取代一个或多个卤素取代的较低烷氧基团;n是1到6之间的整数;X3是有机磺酰氧基团。
-
[EN] METHOD OF PRODUCING AMINOPHENOL COMPOUNDS<br/>[FR] PROCEDE DE FABRICATION DE COMPOSES AMINOPHENOLS申请人:OTSUKA PHARMA CO LTD公开号:WO2005092832A1公开(公告)日:2005-10-06The present invention provides an industrially advantageous method of producing aminophenol compounds represented by the formula (1) by a simple and easy procedure at a high yield and a high purity. The present invention provides a method of producing an aminophenol compound represented by the formula (1): (wherein each of R1 and R2, which may be the same or different, is a hydrogen atom, a substituted or unsubstituted lower alkyl group or the like; R1 and R2, taken together with the adjacent nitrogen atom, may form a 5- or 6-membered heterocycle with or without other intervening heteroatoms; the heterocycle may be substituted by 1 to 3 substituents selected from the group consisting of a hydroxyl group, a substituted or unsubstituted lower alkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted aryloxy group and the like; and the hydroxyl group in the formula (1) is substituted on the 2- or 4-position to the amino group on the phenyl ring), which comprises allowing a cyclohexanedione compound represented by the formula (2) to react with an amine compound represented by the formula (3) (wherein R1 and R2 are as defined above), under a neutral or basic condition.本发明提供一种在高产率和高纯度下通过简单易行的程序生产由式(1)表示的氨基酚化合物的工业上有利的方法。本发明提供了一种生产由式(1)表示的氨基酚化合物的方法:(其中R1和R2中的每一个,可能相同也可能不同,是氢原子,取代或未取代的较低烷基基团或类似物;R1和R2与相邻氮原子一起可以形成5或6成员的杂环,有或无其他插入的杂原子;该杂环可以被1至3个取代基取代,所述取代基选自羟基团、取代或未取代的较低烷基基团、取代或未取代的芳基基团、取代或未取代的芳氧基团等;并且在式(1)中的羟基团被取代在苯环上的氨基团的2-或4-位置),其包括使由式(2)表示的环己二酮化合物与由式(3)表示的胺化合物(其中R1和R2如上定义)在中性或碱性条件下反应。
-
PROCESS FOR PRODUCING BENZO[B][1,4]DIAZEPINE-2,4-DIONE COMPOUND申请人:Tsujimori Hisayuki公开号:US20120149899A1公开(公告)日:2012-06-14The present invention provides an industrially advantageous, simple, and efficient process for producing a key intermediate of a benzo[b][1,4]diazepine-2,4-dione compound, which is a therapeutic medicine for arrhythmia. The present invention relates to a process for producing a compound represented by formula (1), wherein each of R 1 , R 2 , R 3 and R 4 , which may be the same or different, represents a hydrogen atom or a lower alkyl group, the process including deprotecting a protective group (R 5 ) of a compound represented by formula (2), wherein R 1 , R 2 , R 3 and R 4 are as defined above, and R 5 represents a protective group of a hydroxy group.本发明提供了一种在工业上具有优势的、简单而高效的生产苯并[b][1,4]二氮杂环己二酮化合物的关键中间体的方法,该化合物是心律失常的治疗药物。本发明涉及一种生产由式(1)表示的化合物的方法,其中R1、R2、R3和R4中的每一个,可以相同也可以不同,表示氢原子或低碳基团,该方法包括去保护式(R5)的步骤,该保护式是由式(2)表示的化合物的保护基,其中R1、R2、R3和R4如上定义,R5表示羟基的保护基。
-
DIARYLETHER DERIVATIVES AS ANTITUMOR AGENTS申请人:Matsuyama Hironori公开号:US20100004438A1公开(公告)日:2010-01-07An object of the present invention is to provide a medicinal drug much improved in anti tumor activity and excellent in safety. According to the present invention, there is provided a medicinal drug containing a compound represented by the following general formula (1) or a salt thereof as an active ingredient: [Formula 1] wherein X 1 represents a nitrogen atom or a group —CH═, R 1 represents a group -Z-R 6 , in which Z represents a group —CO—, a group —CH(OH)— or the like, R 6 represents a 5- to 15-membered monocyclic, dicyclic or tricyclic saturated or unsaturated heterocyclic group having 1 to 4 nitrogen atoms, oxygen atoms or sulfur atoms, R 2 represents a hydrogen atom or a halogen atom, Y represents a group —O—, a group —CO—, a group —CH(OH)— or a lower alkylene group, and A represents [Formula 2] wherein R 3 represents a hydrogen atom, a lower alkoxy group or the like, p represents 1 or 2, R 4 represents an imidazolyl lower alkyl group or the like.
表征谱图
-
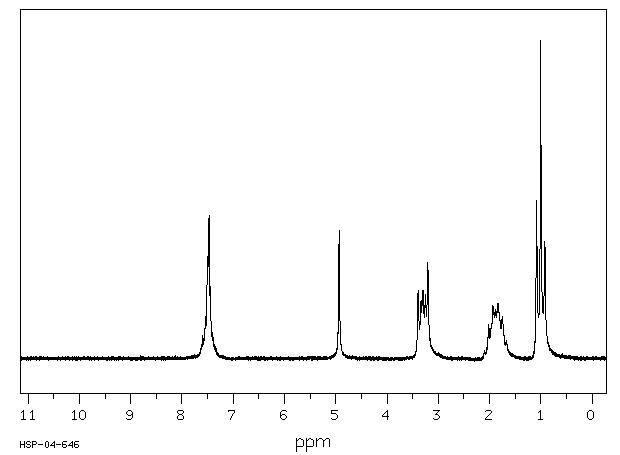
氢谱1HNMR
-
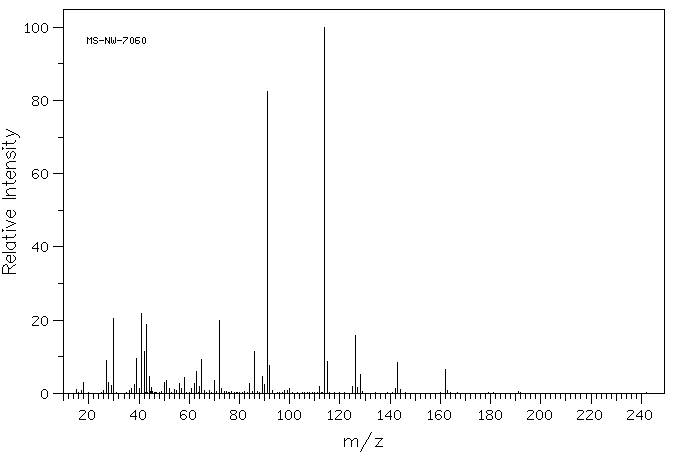
质谱MS
-
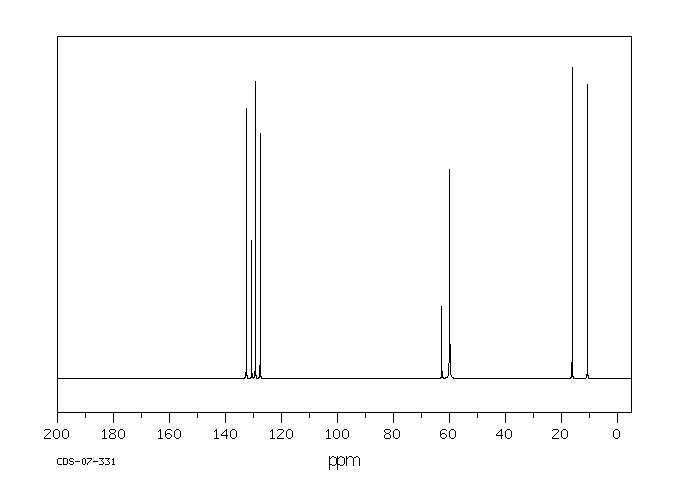
碳谱13CNMR
-
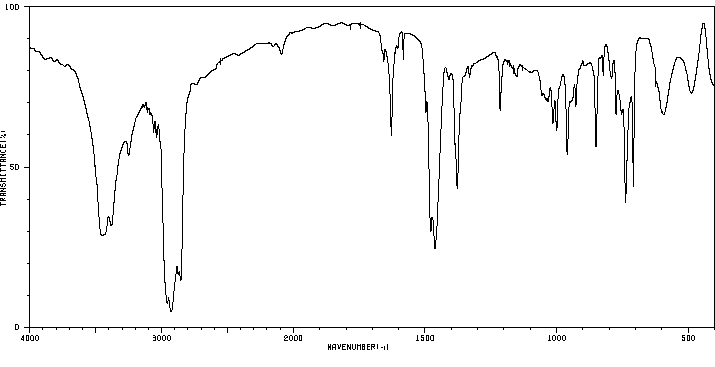
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫