4-氨基-N,N-二甲基苯甲酰胺 | 6331-71-1
中文名称
4-氨基-N,N-二甲基苯甲酰胺
中文别名
——
英文名称
4-Amino-N,N-dimethylbenzamide
英文别名
——
CAS
6331-71-1
化学式
C9H12N2O
mdl
MFCD00523648
分子量
164.207
InChiKey
QEPGWLBMAAEBCP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:151-154°C
-
沸点:348.0±25.0 °C(Predicted)
-
密度:1.116±0.06 g/cm3 (20 ºC 760 Torr)
-
稳定性/保质期:
常温常压下稳定,为棕色液体。
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:46.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2924299090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将药品存放在避光、阴凉干燥的地方,并密封保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
N,N-Dimethyl 4-aminobenzamide
Product Name:
Synonyms: 4-Amino-n,n-dimethylbenzamide
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
N,N-Dimethyl 4-aminobenzamide
Ingredient name:
CAS number: 6331-71-1
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H12N2O
Molecular weight: 164.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
N,N-Dimethyl 4-aminobenzamide
Product Name:
Synonyms: 4-Amino-n,n-dimethylbenzamide
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
N,N-Dimethyl 4-aminobenzamide
Ingredient name:
CAS number: 6331-71-1
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H12N2O
Molecular weight: 164.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基-4-硝基苯甲酰胺 N,N-dimethyl-4-nitrobenzamide 7291-01-2 C9H10N2O3 194.19 4-氯-N,N-二甲基苯甲酰胺 N,N-dimethyl-4-chlorobenzamide 14062-80-7 C9H10ClNO 183.637 —— tert-butyl (4-(dimethylcarbamoyl)phenyl)carbamate 1016745-07-5 C14H20N2O3 264.324 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-hydrazino-N,N-dimethyl-benzamide 806634-16-2 C9H13N3O 179.222 —— 4-isothiocyanato-N,N-dimethylbenzamide 1192762-12-1 C10H10N2OS 206.268 4-(己基氨基)-N,N-二甲基苯甲酰胺 4-(hexylamino)-N,N-dimethylbenzamide 1001083-36-8 C15H24N2O 248.368 —— 4-(acryloylamino)-N,N-dimethylbenzamide 145129-71-1 C12H14N2O2 218.255 4-(二甲基氨甲基)苯胺 4-(dimethylaminomethyl)aniline 6406-74-2 C9H14N2 150.224 4-[二(2-氯乙基)氨基]-N,N-二甲基苯甲酰胺 Benzamide, 4-(bis(2-chloroethyl)amino)-N,N-dimethyl- 122567-50-4 C13H18Cl2N2O 289.205 —— 4-{[(2-Chloroethyl)carbamoyl]amino}-n,n-dimethylbenzamide 13908-49-1 C12H16ClN3O2 269.731 —— 4-(5-amino-1H-[1,2,4]triazol-3-ylamino)-N,N-dimethylbenzamide 1533424-15-5 C11H14N6O 246.272
反应信息
-
作为反应物:描述:参考文献:名称:硼酸催化的氢化硅烷化将酰胺选择性还原为胺摘要:不是'B'ore!在存在氢硅烷的情况下,基于苯并噻吩的硼酸催化叔,仲和伯酰胺的还原。该反应显示出良好的官能团耐受性。DOI:10.1002/anie.201304495
-
作为产物:描述:tert-butyl (4-(dimethylcarbamoyl)phenyl)carbamate 在 盐酸 作用下, 以 乙酸乙酯 为溶剂, 反应 5.0h, 以615 mg的产率得到4-氨基-N,N-二甲基苯甲酰胺参考文献:名称:DERIVATIVES OF 1-PHENYL-2-PYRIDINYL ALKYL ALCOHOLS AS PHOSPHODIESTERASE INHIBITORS摘要:根据公式(I),1-苯基-2-吡啶基烷基醇的衍生物可用作磷酸二酯酶4(PDE4)酶的抑制剂。公开号:US20130324501A1
文献信息
-
Copper catalyzed cross-coupling reactions of carboxylic acids: an expedient route to amides, 5-substituted γ-lactams and α-acyloxy esters作者:S. Priyadarshini、P. J. Amal Joseph、M. Lakshmi KantamDOI:10.1039/c3ra41000e日期:——A convenient and recyclable catalytic protocol for the synthesis of N,N-dimethyl substituted amides, 5-substituted γ-lactams and α-acyloxy ethers from carboxylic acids using CuO nanoparticles and TBHP is described.
-
[EN] COMPOUNDS AND COMPOSITIONS FOR TREATING CONDITIONS ASSOCIATED WITH NLRP ACTIVITY<br/>[FR] COMPOSÉS ET COMPOSITIONS DESTINÉS AU TRAITEMENT D'ÉTATS PATHOLOGIQUES ASSOCIÉS À UNE ACTIVITÉ NLRP申请人:IFM TRE INC公开号:WO2019023145A1公开(公告)日:2019-01-31In one aspect, compounds of Formula AA, or a pharmaceutically acceptable salt thereof, are featured. The variables shown in Formula AA are as defined in the claims. The compounds of formula AA are NLRP3 activity modulators and, as such, can be used in the treatment of metabolic disorders (e.g. Type 2 diabetes, atherosclerosis, obesity or gout), a disease of the central nervous system (e.g. Alzheimer's disease, multiple sclerosis, Amyotrophic Lateral Sclerosis or Parkinson's disease), lung disease (e.g. asthma, COPD or pulmonary idiopathic fibrosis), liver disease (e.g. NASH syndrome, viral hepatitis or cirrhosis), pancreatic disease (e.g. acute pancreatitis or chronic pancreatitis), kidney disease (e.g. acute kidney injury or chronic kidney injury), intestinal disease (e.g. Crohn's disease or Ulcerative Colitis), skin disease (e.g. psoriasis), musculoskeletal disease (e.g. scleroderma), a vessel disorder (e.g. giant cell arteritis), a disorder of the bones (e.g. osteoarthritis, osteoporosis or osteopetrosis disorders), eye disease (e.g. glaucoma or macular degeneration), a disease caused by viral infection (e.g. HIV or AIDS), an autoimmune disease (e.g. Rheumatoid Arthritis, Systemic Lupus Erythematosus or Autoimmune Thyroiditis), cancer or aging.在一个方面,特色化合物AA的公式,或其药学上可接受的盐。在公式AA中显示的变量如索引中所定义。公式AA的化合物是NLRP3活性调节剂,因此可用于治疗代谢性疾病(例如2型糖尿病、动脉粥样硬化、肥胖或痛风)、中枢神经系统疾病(例如阿尔茨海默病、多发性硬化症、肌萎缩性侧索硬化或帕金森病)、肺部疾病(例如哮喘、慢性阻塞性肺疾病或肺部特发性纤维化)、肝脏疾病(例如NASH综合征、病毒性肝炎或肝硬化)、胰腺疾病(例如急性胰腺炎或慢性胰腺炎)、肾脏疾病(例如急性肾损伤或慢性肾损伤)、肠道疾病(例如克罗恩病或溃疡性结肠炎)、皮肤疾病(例如牛皮癣)、肌肉骨骼疾病(例如硬皮病)、血管障碍(例如巨细胞动脉炎)、骨骼疾病(例如骨关节炎、骨质疏松症或骨质增生疾病)、眼部疾病(例如青光眼或黄斑变性)、病毒感染引起的疾病(例如HIV或艾滋病)、自身免疫疾病(例如类风湿性关节炎、系统性红斑狼疮或自身免疫性甲状腺炎)、癌症或衰老。
-
Novel Compounds for the Treatment of Diseases Associated with Amyloid or Amyloid-Like Proteins申请人:Kroth Heiko公开号:US20110280808A1公开(公告)日:2011-11-17The present invention relates to novel compounds that can be employed in the treatment of a group of disorders and abnormalities associated with amyloid protein, such as Alzheimer's disease, and of diseases or conditions associated with amyloid-like proteins. The compounds of the present invention can also be used in the treatment of ocular diseases associated with pathological abnormalities/changes in the tissues of the visual system. The present invention further relates to pharmaceutical compositions comprising these compounds and to the use of these compounds for the preparation of medicaments for treating or preventing diseases or conditions associated with amyloid and/or amyloid-like proteins. A method of treating or preventing diseases or conditions associated with amyloid and/or amyloid-like proteins is also disclosed.本发明涉及一种新型化合物,可用于治疗与淀粉样蛋白相关的一组疾病和异常,如阿尔茨海默病,以及与淀粉样蛋白类似蛋白相关的疾病或病况。本发明的化合物还可用于治疗与视觉系统组织中的病理异常/变化相关的眼部疾病。本发明还涉及包含这些化合物的药物组合物,以及利用这些化合物制备用于治疗或预防与淀粉样和/或淀粉样类似蛋白相关的疾病或病况的药物的用途。还公开了一种治疗或预防与淀粉样和/或淀粉样类似蛋白相关的疾病或病况的方法。
-
[EN] PYRIMIDINE DERIVATIVES CAPABLE OF INHIBITING ONE OR MORE KINASES<br/>[FR] DÉRIVÉS DE PYRIMIDINE CAPABLES D'INHIBER UNE OU PLUSIEURS KINASES申请人:MEDICAL RES COUNCIL公开号:WO2009122180A1公开(公告)日:2009-10-08A first aspect of the invention relates to a compound of formula (I), or a pharmaceutically acceptable salt or ester thereof, formula (I): wherein: R1 is C3-8-cycloalkyl; X is O, NR7 or C3-6-heterocycloalkyl; R2 is aryl, heteroaryl, fused or unfused aryl-C3-6-heterocycloalkyl or fused or unfused heteroaryl-C3-6-heterocycIoalkyl, each of which is optionally substituted by one or more substitutents selected from aryl, heteroaryl, C1-6-alkyl, C3-7-cycloalkyl and a group A, wherein said C1-6-alkyl group is optionally substituted by one or more substituents selected from aryl, heteroaryl, R10 and a group A, said heteroaryl group is optionally substituted by one or more R10 groups; and wherein said C3-6-heterocycloalkyl group optionally contains one or more groups selected from oxygen, sulfur, nitrogen and CO; R3 is C1-6-alkyl optionally substituted by one or more substituents selected from aryl, heteroaryl, -NR4R5, -OR6, -NR7(CO)R6, -NR7(CO)NR4R5, -NR7SO2R6, -NR7COOR7, -CONR4R5, C3-6-heterocycloalkyl and formula (a, b, c): wherein R4-7 and A are as defined in the claims. Further aspects relate to the use of said compounds in the treatment of various therapeutic disorders, and more particularly as inhibitors of one or more kinases.本发明的第一个方面涉及式(I)的化合物,或其药学上可接受的盐或酯,式(I)如下:其中:R1为C3-8环烷基;X为O、NR7或C3-6杂环烷基;R2为芳基、杂芳基、融合或未融合的芳基-C3-6杂环烷基或融合或未融合的杂芳基-C3-6杂环烷基,每个基可选择地由来自芳基、杂芳基、C1-6烷基、C3-7环烷基和A基的一个或多个取代基取代,其中所述C1-6烷基基可选择地由来自芳基、杂芳基、R10和A基的一个或多个取代基取代,所述杂芳基可选择地由一个或多个R10基取代;以及所述C3-6杂环烷基基可选择地包含一个或多个来自氧、硫、氮和CO的基;R3为C1-6烷基,可选择地由一个或多个来自芳基、杂芳基、-NR4R5、-OR6、-NR7(CO)R6、-NR7(CO)NR4R5、-NR7SO2R6、-NR7COOR7、-CONR4R5、C3-6杂环烷基和式(a, b, c)的取代基取代;其中R4-7和A如权利要求中所定义。进一步方面涉及所述化合物在治疗各种治疗性疾病中的使用,特别是作为一个或多个激酶的抑制剂。
-
2-Arylamino-6-ethynylpurines are cysteine-targeting irreversible inhibitors of Nek2 kinase作者:Christopher J. Matheson、Christopher R. Coxon、Richard Bayliss、Kathy Boxall、Benoit Carbain、Andrew M. Fry、Ian R. Hardcastle、Suzannah J. Harnor、Corine Mas-Droux、David R. Newell、Mark W. Richards、Mangaleswaran Sivaprakasam、David Turner、Roger J. Griffin、Bernard T. Golding、Céline CanoDOI:10.1039/d0md00074d日期:——Renewed interest in covalent inhibitors of enzymes implicated in disease states has afforded several agents targeted at protein kinases of relevance to cancers. We now report the design, synthesis and biological evaluation of 6-ethynylpurines that act as covalent inhibitors of Nek2 by capturing a cysteine residue (Cys22) close to the catalytic domain of this protein kinase. Examination of the crystal人们对与疾病状态有关的酶的共价抑制剂重新产生了兴趣,从而提供了几种针对与癌症相关的蛋白激酶的药物。我们现在报告了 6-乙炔基嘌呤的设计、合成和生物学评价,6-乙炔基嘌呤通过捕获靠近该蛋白激酶催化结构域的半胱氨酸残基 (Cys22) 来充当 Nek2 的共价抑制剂。对与 Nek2 复合的非共价抑制剂 3-((6-环己基甲氧基-7 H-嘌呤-2-基)氨基)苯甲酰胺的晶体结构的检查表明,用乙炔基取代烷氧基放置了炔烃的末端接近Cys22并且处于与迈克尔加成的立体电子学要求兼容的位置。制备了一系列 6-乙炔基嘌呤并建立了抑制 Nek2 的结构活性关系 (SAR)。 6-乙炔基-N-苯基-7 H-嘌呤-2-胺 [IC 50 0.15 μM (Nek2)] 和 4-((6-乙炔基-7 H-嘌呤-2-基)氨基)苯磺酰胺 (IC 50 0.14)选择 Nek2 的抑制模式来确定 Nek2 的抑制模式,该抑制模式具有时间依赖性,不能通过添加
表征谱图
-
氢谱1HNMR
-
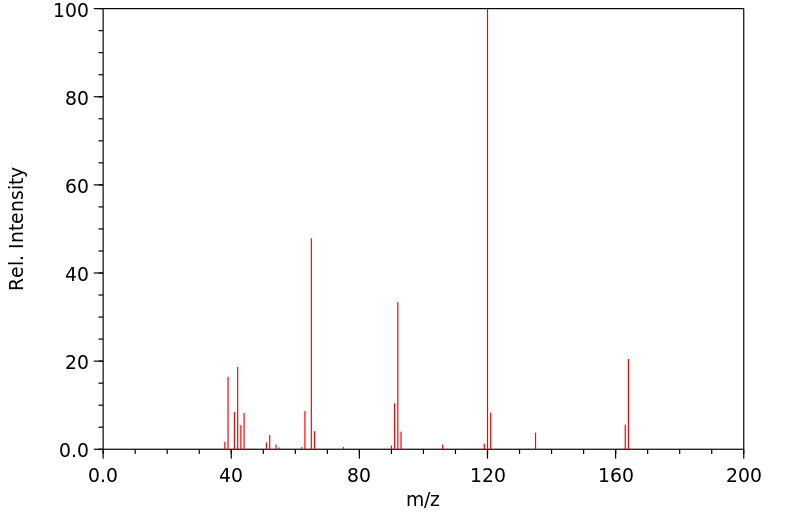
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫