2-乙基-1,3-二噻烷 | 6007-23-4
中文名称
2-乙基-1,3-二噻烷
中文别名
——
英文名称
2-ethyl-1,3-dithiane
英文别名
——
CAS
6007-23-4
化学式
C6H12S2
mdl
MFCD12910519
分子量
148.293
InChiKey
MMCSAFCIQDJGDY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:116°C/22mmHg
-
保留指数:1131;1129
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,3-二噻烷 1,3 dithiane 505-23-7 C4H8S2 120.24
反应信息
-
作为反应物:描述:2-乙基-1,3-二噻烷 在 titanium(IV) isopropylate 、 叔丁基过氧化氢 、 正丁基锂 、 L-(+)-酒石酸二乙酯 、 水 、 二甲基亚砜 、 三乙胺 、 三氟乙酸酐 作用下, 以 二氯甲烷 为溶剂, 反应 145.0h, 生成 anti-2-acetyl-2-ethyl-1,3-dithiane 1R-oxide参考文献:名称:使用改良的无尖硫氧化方法对映选择性制备2-取代的1,3-二硫杂环丁烷1-氧化物摘要:已经使用改良的Sharpless条件进行了范围广泛的2-取代的1,3-二硫杂环丁烷的对映选择性磺氧化,以提供光学富集形式的相应亚砜。2-酰基-1,3-二硫杂环丁烷1-氧化物衍生物的脱酰作用可以制备2-烷基-1,3-二硫杂环丁烷1-氧化物和母体1,3-二硫杂环丁烷1-氧化物本身以高对映体过量的方式制备。DOI:10.1016/0040-4020(95)01029-7
-
作为产物:描述:2-ethyl-5,6-dihydro-4H-1,3-dithiin-1-ium tetrafluoroborate 在 sodium tetrahydroborate 作用下, 以 乙腈 为溶剂, 以57%的产率得到2-乙基-1,3-二噻烷参考文献:名称:Stahl, Ingfried, Chemische Berichte, 1985, vol. 118, # 8, p. 3166 - 3171摘要:DOI:
文献信息
-
TRANS-3,5-DISUBSTITUTEDPYRROLIDINE: ORGANOCATALYST FOR anti-MANNICH REACTIONS申请人:Tanaka Fujie公开号:US20070117986A1公开(公告)日:2007-05-24A compound of Formula I is disclosed, in which R is a substituent containing a hydrogen bond-forming atom within three atoms from the ring carbon to which the substituent is bonded; X is CH 2 , O, S or NR 1 , wherein R 1 is a hydrocarbyl group or an amino-protecting group having one to about 18 carbon atoms; R 2 is hydrido or a hydrocarbyl group containing one to about twelve carbon atoms; and R 3 is hydrido or methyl, but both R 2 and R 3 are not hydrido when X is CH 2 A molecule of Formula I and those in which R 2 and R 3 can both be hydrido (Formula X) functions as a catalyst in a Mannich reaction to asymmetrically form β-aminoaldehyde or β-aminoketone diastereomeric products having two chiral centers on adjacent carbon atoms and in which the anti-diastereomers are in excess over the syn-diastereomers. Methods for carrying out those syntheses are also disclosed.
-
Asymmetric Aza-Wacker-Type Cyclization of <i>N</i>-Ts Hydrazine-Tethered Tetrasubstituted Olefins: Synthesis of Pyrazolines Bearing One Quaternary or Two Vicinal Stereocenters作者:Xuezhen Kou、Qihang Shao、Chenghao Ye、Guoqiang Yang、Wanbin ZhangDOI:10.1021/jacs.8b02865日期:2018.6.20aza-Wacker-type cyclization of N-Ts hydrazine-tethered tetrasubstituted olefins, affording optically active pyrazolines bearing chiral tetrasubstituted carbon stereocenters. This reaction is tolerant to a broad range of substrates under mild reaction conditions, giving the desired chiral products with high enantioselectivities. Generation of two vicinal stereocenters on the C═C double bonds was also achieved我们开发了 N-Ts 肼系四取代烯烃的不对称氮杂-瓦克型环化,提供带有手性四取代碳立体中心的旋光吡唑啉。该反应在温和的反应条件下对多种底物具有耐受性,从而得到具有高对映选择性的所需手性产物。在 C=C 双键上的两个邻位立体中心的生成也以高选择性实现,这一过程很少被研究用于瓦克型反应。一项机理研究表明,这种氮杂-瓦克型环化经历了一个顺氨基钯化过程。还发现对于在烯烃的外碳原子上带有两个线性烷基取代基的底物,这两个取代基都大于甲基,亲核内基团顺式的烷基取代基更容易参与β-氢化物的消除。当烯烃外层碳原子上的两个烷基取代基之一为甲基时,β-氢化物消去选择性地在亚甲基侧进行,因此两种非对映异构体都可以通过改变烯烃的构型来制备。此外,该产品可以通过三个步骤以高产率转化为药物化合物。
-
Tetrazole derivatives, and anti-ulcer composition containing the same申请人:Otsuka Pharmaceutical Company, Limited公开号:US04372953A1公开(公告)日:1983-02-08Tetrazole derivatives of the formula: ##STR1## wherein R.sup.1 is a lower alkykl, phenyl or a group of the formula: --S(O).sub.l --A--(X).sub.m --R.sup.3, and R.sup.2 is hydrogen, a lower alkyl, phenyl or a cycloalkyl when R.sup.1 is the group --S(O).sub.l --A--(X).sub.m --R.sup.3, or R.sup.2 is a group of the formula: --B--CO--R.sup.4 when R.sup.1 is a lower alkyl or phenyl and a pharmaceutically acceptable salt thereof, which have prophylactic or therapeutic activities against peptic and/or duodenal ulcers and are useful as an anti-ulcer drug; processes for the preparation of the tetrazole derivatives; and pharmaceutical composition containing said tetrazole derivatives.Tetrazole衍生物的化学式为:##STR1## 其中R.sup.1是较低的烷基、苯基或化学式的基团:--S(O).sub.l --A--(X).sub.m --R.sup.3,而R.sup.2是氢、较低的烷基、苯基或环烷基,当R.sup.1是基团--S(O).sub.l --A--(X).sub.m --R.sup.3时,或者R.sup.2是化学式的基团:--B--CO--R.sup.4,当R.sup.1是较低的烷基或苯基时,以及其药学上可接受的盐,具有预防或治疗胃溃疡和/或十二指肠溃疡的活性,并可用作抗溃疡药物;制备Tetrazole衍生物的方法;以及含有上述Tetrazole衍生物的药物组合物。
-
When Ethyl Is Infinitely Different from Methyl: Double Addition of Lithiated Dithianes to Aromatic Carboxylates Revisited作者:Roman A. Valiulin、Rudresha Kottani、Andrei G. KutateladzeDOI:10.1021/jo060780f日期:2006.6.1benzoate does not produce the expected product of double addition, α,α-bis(alkyldithianyl) benzyl alcohol, for alkyls larger than methyl. Instead, the first step intermediate, i.e. 2-benzoylated dithiane, undergoes an electron-transfer reduction by the second molecule of the dithianyl anion. This reduction is followed by the ring-opening mesolytic fragmentation of the dithiane ring in the ketyl anion radical
-
A Simple and Convenient Catalytic Procedure for the Preparation of Dithioacetals作者:Andrew E. GrahamDOI:10.1080/00397919908085819日期:1999.2Abstract A simple procedure for the synthesis of cyclic and acyclic thioacetals from aldehydes is described which employs hydrogen chloride solutions generated from acetyl chloride and methanol. It was observed that the reaction could be carried out using catalytic quantities of acetyl chloride, potentially allowing acid sensitive substrates to be used under these conditions.
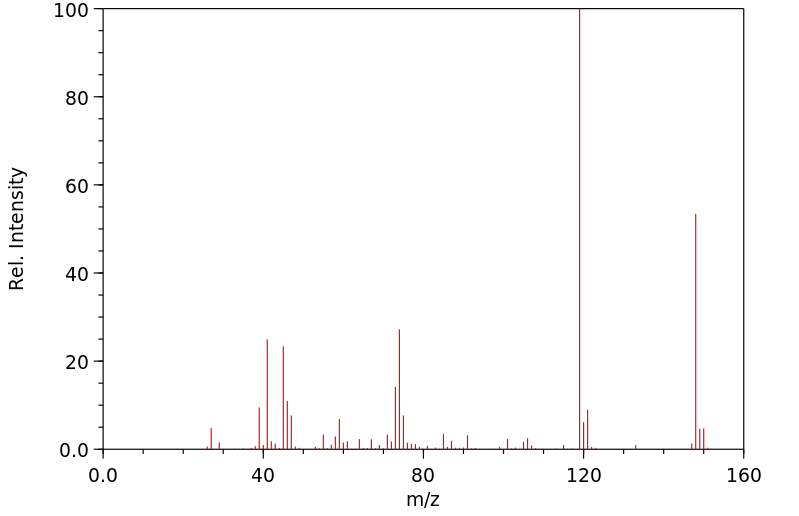
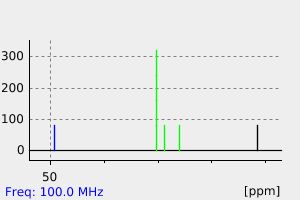
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
硫化膦,1,3-二硫烷-2-基甲基苯基-
硅烷,三甲基(2-甲基-1,3-二硫烷-2-基)-
沙丙喋呤中间体
四氢-1,2-二噻英
反式-1,2-二噻烷-4,5-二醇1,1-二氧化物
八氟-1,4-二噻烷
二(1,3-二噻烷-2-基)甲烷-D
二(1,3-二噻烷-2-基)甲烷
丁二腈,2,3-二[(1,1-二甲基乙基)硫代]-2,3-二(1,3-二硫烷-2-基甲基)-
N-乙基-1,3-二噻烷-2-亚胺
N-(1,3-二硫杂环戊-2-亚基)氨基磷酸二甲酯
N,N’-1,6-己烷二基双氨基甲酸双(1,3-二噻烷-2-基甲基)酯
5alpha-[N-(亚硝基氨基甲酰)-N-(2-氯乙基)氨基]-2beta-甲基-1,3-二噻烷1,1,3,3-四氧化物
5,6-二氢-4H-1,3-二噻英-2-硫酮
4-甲基-2,6,7-三硫杂二环[2.2.2]辛烷
4-(丙氧基甲基)-2,6,7-三硫杂二环[2.2.2]辛烷
3-(1,3-二噻烷-5-基)-1-(2-氟乙基)-1-亚硝基脲
3-(1,3-二噻烷-2-亚基)-2,4-戊二酮
3,3-二甲基二环[2.2.1]庚烷-2-甲醇
2-苯基-1,3-二噻烷锂盐
2-苯基-1,3-二噻烷
2-脱氧-D-阿拉伯糖-己糖亚丙基二硫代缩醛
2-甲基-1,3-二噻烷
2-戊基-1,3-二噻烷
2-异丙基-1,3-二噻烷
2-异丁基-1,3-二噻烷
2-乙炔基-1,3-二噻烷
2-乙基-1,3-二噻烷
2-三甲基硅基-1,3-二噻吩
2-(叔丁基二甲基甲硅烷基)-1,3-二噻烷
2-(三异丙基甲硅烷基)-1,3-二噻烷
2-(3,4-二羟基苯基)-5,7-二羟基-6-[(2S,3R,4R,5S,6R)-3,4,5-三羟基-6-(羟甲基)四氢-2H-吡喃-2-基]-8-[(2S,3R,4S,5S)-3,4,5-三羟基四氢-2H-吡喃-2-基]-4H-色烯-4-酮(non-preferredname)
2-(1,3-二噻烷-2-基)乙醇
2,5-二甲基-2,5-二羟基-1,4-二噻烷
2,5-二甲基-2,5-二羟基-1,4-二噻烷
2,5-二乙氧基-1,4-二噻烷
2,2’-乙烯双(1,3-二噻烷)
2,2-双(三甲基硅基)二噻烷
2,2-二氟-1,3-二噻烷
2,2'-(1,2-亚苯基)二(1,3-二噻烷)
1-(2-氯乙基)-3-(2alpha-甲基-1,3-二噻烷-5alpha-基)-3-亚硝基脲
1-(2-氯乙基)-3-(1,3-二噻烷-5-基)-1-亚硝基脲
1-(2-氯乙基)-1-亚硝基-3-(1,1,3,3-四氧代-1,3-二噻烷-5-基)脲
1-(2-氟乙基)-1-亚硝基-3-(1,1,3,3-四氧代-1,3-二噻烷-5-基)脲
1-(1,3-二噻烷-2-基)乙酮
1-(1,3-二噻烷-2-基)-2-环己烯-1-醇
1-(1,3-二噻烷-2-基)-2,2,2-三氟乙烷酮
1,8-二羟基-2,9-二硫杂三环[8.4.0.03,8]十四烷
1,5,7,11-四硫杂螺[5.5]十一烷
1,4-苯并二噻英,八氢-