十四氟-1-庚烯 | 355-63-5
中文名称
十四氟-1-庚烯
中文别名
全氟庚烯-1
英文名称
perfluoro-1-heptene
英文别名
Perfluorohept-1-ene;1,1,2,3,3,4,4,5,5,6,6,7,7,7-tetradecafluorohept-1-ene
CAS
355-63-5
化学式
C7F14
mdl
MFCD00013577
分子量
350.055
InChiKey
CDAVUOSPHHTNBU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:80°C
-
密度:1.8748 (rough estimate)
-
保留指数:312
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:21
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:14
安全信息
-
危险等级:3.1
-
危险品标志:Xi
-
安全说明:S23,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2903399090
-
包装等级:II
-
危险类别:3.1
-
危险品运输编号:1992
-
储存条件:请保持冷静。
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Preparation of β-H-Perfluoro Alkanesulfonic Acids摘要:DOI:10.1021/ja01114a517
-
作为产物:描述:参考文献:名称:源自三氟氯乙烯的液体。一,模型化合物的合成摘要:制备了一系列在相邻碳原子上具有氯的氯氟烷烃,即nC 5 F 11 CFClCCl 3,nC 6 F 13 CFClCCl 3,nC 5 F 11 CFClCFCl 2,nC 6 F 13 CFClCFCl 2,nC 5 F 11 CFClCF 2 Cl ,nC 2 F 5 CFClCFClC 3 F 7和n-CF 3 CFClCFClC 4 F 9通过卤素交换,脱卤和氯加成反应的组合。在存在汞的情况下,通过在紫外线辐射下将nC 6 F 13 CFClI与过量的ICF 2 CFCl 2偶联来合成nC 6 F 13 CFClCF 2 CFCl 2的尝试,仅给出了nC 6 F 13 CFClCFClCC 6 F 13和CFCl的合成。2 CF 2 CF 2 CFCl 2。在nC 6 F 13 CFClI和CF 2的平行条件下以48%的产率获得ClCFClI,n-C 6 F 13 CFClCFClCFDOI:10.1016/s0022-1139(00)82355-6
文献信息
-
Interaction of perfluorocarbons with carbon作者:F.J. WeigertDOI:10.1016/s0022-1139(00)80475-3日期:1993.11fluorine acceptor which defluorinates perfluoroalkanes with the partial structure (Rf)2CFCF(Rf)2 to alkenes and perfluoroalkenes to dienes. Carbon catalyzes double-bond shifts, as well as cis/trans and ring-chain perfluoro-olefin isomerizations. Carbon effectively catalyzes TFE and HFP dimerizations. Less effectively, carbon catalyzes further oligomerization of TFE to give low yields of linear, internal olefins
-
[3 + 2]-Dipolar Cycloaddition Reactions of an N-Heterocyclic Carbene Boryl Azide作者:Everett Merling、Vladimir Lamm、Steven J. Geib、Emmanuel Lacôte、Dennis P. CurranDOI:10.1021/ol300851m日期:2012.6.1Thermal 1,3-dipolar cycloaddition reactions of 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene dihydridoboron azide occur smoothly with alkynes, nitriles, and alkenes bearing electron-withdrawing groups. New, stable NHC-boryl-substituted triazoles, tetrazoles, and triazolidines are formed in good to excellent yields.
-
Radical additions to fluoroolefins. Thermal reaction of perfluoroallyl chloride with perfluoroalkyl iodides as a selective synthesis of terminal perfluoroolefins作者:Vladimír Církva、Oldřich Paleta、Bruno Ameduri、Bernard BoutevinDOI:10.1016/0022-1139(95)03202-o日期:1995.11Reaction of perfluoroallyl chloride with perfluoroalkyl iodides, RFI (RF = C4F9, C6F13, C8F17), in an autoclave at 180–250 °C gave terminal perfluoroolefins, CF2-CF-CF2-RF (2–4) as the sole reaction products. Isomeric fluoroolefins containing an internal double bond and telomeric products were not observed in the reaction mixture. The yields were dependent on the reaction temperature and on the chain全氟烯丙基氯与全氟烷基碘R F I(R F = C 4 F 9,C 6 F 13,C 8 F 17)在高压釜中于180–250°C反应,得到末端全氟烯烃CF 2 -CF-CF 2 -R F(2-4)作为唯一反应产物。在反应混合物中未观察到含有内部双键的异构氟代烯烃和端粒产物。收率取决于反应温度和R F I的链长:烯烃2(R F在200°C(26%)下获得= C 4 F 9),而在烯烃3和4(分别为R F = C 6 F 13和C 8 F 17)的合成中,在250°C下获得最高收率(分别为41%和74%)。CuI或过氧化物引发剂的存在对产物的产率具有负面影响。在产物2–4的质谱图中,已将公式指定给碎片,并已提出了碎片序列。
-
Electrophilic versus free radical reactions of halogens and halogen systems with perfluoroalkenes and a perfluoroalkylethylene作者:Dale F Shellhamer、Jeannette L Allen、Rachel D Allen、Melissa J Bostic、Elizabeth A Miller、Colleen M O’Neill、Benjamin J Powers、Eric A Price、John W Probst、Victor L HeasleyDOI:10.1016/s0022-1139(00)00319-5日期:2000.10Alkene 3 is more reactive and gives products with electrophiles Cl2, Br2, BrCl, and ICl from ring-opening of the halonium ions at the terminal carbon. Disubstituted perfluoroalkenes 2 and 4 did not react ionically with Cl2, Br2, BrCl, or ICl. Free radical reactions of Cl2, Br2, BrCl, and ICl give good yields of dihaloproducts with 1 and 3. Yields are poor for the photochemical bromination of disubstituted用全氟庚烯-1(1),八氟环戊烯(2),1H,1H,2H-全氟辛烯-1(3)和全氟-4-甲基-2-戊烯(4)进行卤素系统的离子和自由基加成。在确保离子路径的反应条件下,用汞催化剂在叔丁醇中作为溶剂用氯处理末端烯烃1。溴在类似条件下不会与1反应。一氟化氯与1在二氯甲烷中的反应生成2-氯全氟庚烷。烯烃3更具反应性,并产生具有亲电子试剂Cl 2,Br 2的产物,BrCl和ICl是由于the离子在末端碳原子处的开环而产生的。二取代的全氟烯烃2和4不会与Cl 2,Br 2,BrCl或ICl发生离子反应。Cl 2,Br 2,BrCl和ICl的自由基反应可得到1和3的二卤代产物。双取代的全氟烯烃2和4的光化学溴化收率不佳,因为二溴代产物与烯烃处于光化学平衡。
-
Reaktionen von fluoralkenen I. Zur nucleophilen reaktion von F-hept-1-en mit alkoholen作者:U. Groβ、W. StorekDOI:10.1016/s0022-1139(00)80973-2日期:1984.12The reaction of F-hept-1-ene together with aromatic and aliphatic alcohols has been investigated. In the case of disodiumphenolsulfonate, trans-F-hept-2-enyl-1-oxybenzenesulfonate was formed, exclusively. The structure of this new fluorosurfactant was confirmed by means of 19FNMR. In contrast, phenoxide and alkoxide yield a mixture of products, including the isomers of allylic and vinylic substitution
表征谱图
-
氢谱1HNMR
-
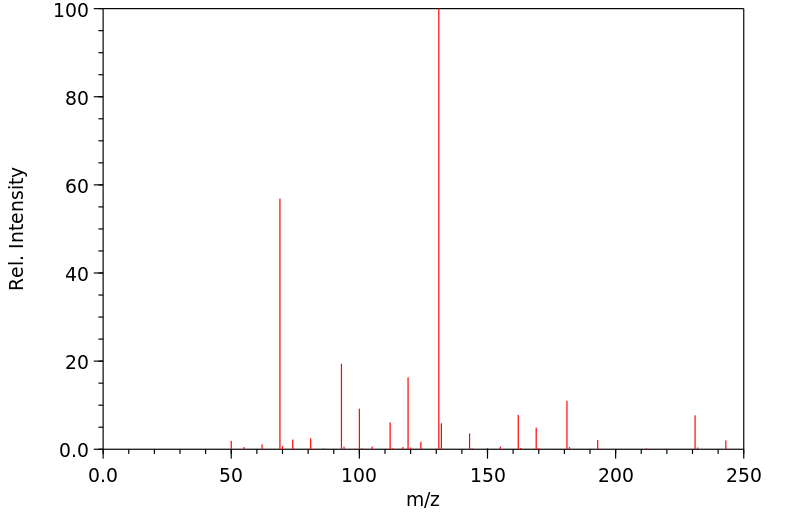
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-3-甲基-1,2,3,4-四氯-1-丁烯
顺式-1-溴-1-丙烯
顺式-1-氯-1-丁烯
顺式-1,3-二氯丙烯
顺式-1,2-二碘乙烯
顺式-1,2-二溴乙烯
顺式-1,2-二氟-1-氯乙烯
顺-氯丹
顺-九氯
顺-九氯
顺-1-溴-2-乙氧基乙烯
顺-1,2-二氯乙烯
顺-1,2,4-三氯-3-甲基-2-丁烯
顺,顺-1,2,3,4-四氯-1,3-丁二烯
除螨灵
锗烷,(1-溴-1,2-丙二烯基)三甲基-
锌,氯(三氟乙烯基)-
铜(1+),1,1,2-三氟乙烯
苯甲酸,4-[(1E)-2-[[(4-氯苯基)甲基]磺酰]乙烯基]-
苯并烯氟菌唑中间体
艾日布林-2碘
聚(乙烯-氯代三氟乙烯)
碳化镁碘化物
碘化乙烯
硫丹醇
硅烷,二氯(2-氯乙烯基)甲基-
硅烷,[2-(碘亚甲基)己基]三甲基-,(Z)-
甲碘乙烯
甲氧基全氟丁烷-反式-1,2-二氯乙烯1:1共沸物
甲基烯丙基溴化镁
甲基全氟-1-甲基-2-丙烯基醚
甲基全氟-1-丁基-1-丙烯基醚
甲基全氟-1-丁基-1-丙烯基醚
环丙烷,1,1-二氯-2-(3,3-二氯-2-甲基-2-丙烯基)-2,3,3-三甲基-
环丙烯,1,2-二氟-
特比萘芬杂质
溴西克林
溴甲基烯酮
溴环辛四烯
溴氯丙烯
溴代三氟代乙烯
溴亚甲基环己烷
溴乙烯
溴三碘乙烯
氰尿酰氟
氯磺酸三氟乙烯基酯
氯化聚乙烯
氯乙烯与异丁基乙烯醚共聚物
氯乙烯与三氯乙烯聚合物
氯乙烯-d3