(E)-1,4-dimethoxy-2-(2-nitroprop-1-en-1-yl)benzene | 134040-27-0
中文名称
——
中文别名
——
英文名称
(E)-1,4-dimethoxy-2-(2-nitroprop-1-en-1-yl)benzene
英文别名
1-(2,5-Dimethoxyphenyl)-2-nitropropene;1,4-dimethoxy-2-[(E)-2-nitroprop-1-enyl]benzene
CAS
134040-27-0
化学式
C11H13NO4
mdl
——
分子量
223.229
InChiKey
MQFREHNTGKNSRH-SOFGYWHQSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:16
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:64.3
-
氢给体数:0
-
氢受体数:4
反应信息
-
作为反应物:描述:(E)-1,4-dimethoxy-2-(2-nitroprop-1-en-1-yl)benzene 在 sodium hydroxide 作用下, 以 四氢呋喃 、 水 为溶剂, 生成 2-氨基-4-苯基丙烷盐酸盐参考文献:名称:Immunoassay for Phenethylamines of the 2C and DO Sub-Families摘要:描述了用于检测和测定2C和DO亚家族苯乙胺的免疫分析方法及其必要组成部分。公开号:US20150038366A1
-
作为产物:描述:参考文献:名称:Immunoassay for Phenethylamines of the 2C and DO Sub-Families摘要:描述了用于检测和测定2C和DO亚家族苯乙胺的免疫分析方法及其必要组成部分。公开号:US20150038366A1
文献信息
-
Synthesis, Antiproliferative and Pro-Apoptotic Effects of Nitrostyrenes and Related Compounds in Burkitt's Lymphoma作者:Andrew J. Byrne、Sandra A. Bright、Darren Fayne、James P. McKeown、Thomas McCabe、Brendan Twamley、Clive Williams、Mary J. MeeganDOI:10.2174/1573406413666171002123907日期:2018.2.6identified as a lead target structure for the development of particularly effective compounds targeting Burkitt's lymphoma (BL). OBJECTIVES The aims of the curent study were to synthesise a panel of nitrostyrene compounds and to evaluate their activity in Burkitt's lymphoma (BL). METHODS A panel of structurally varied compounds were designed and synthesised using Henry Knoevenagel condensation reactions.背景技术淋巴细胞癌(淋巴瘤)约占全世界恶性疾病的12%。硝基苯乙烯支架被认为是开发针对Burkitt淋巴瘤(BL)的特别有效的化合物的主要靶标结构。目的目前的研究目的是合成一组硝基苯乙烯化合物并评估其在伯基特氏淋巴瘤(BL)中的活性。方法使用Henry Knoevenagel缩合反应设计和合成一组结构变化的化合物。单晶X射线分析证实了这些新颖结构的六个实例的E构型。还研究了许多与硝基苯乙烯有关的化合物,包括1,3-双(芳基)-2-硝基丙烯与含有硝基乙烯基药效团的杂环支架,例如3-硝基-2-苯基-2H-色烯。使用BL细胞系EBV-MUTU-1和EBV + DG-75(耐化学性)评估化合物的抗增殖活性,以建立初步的结构活性关系。结果成功建立了具有优化的硝基苯乙烯骨架和3-硝基-2-苯基-2Hchromne结构的铅化合物,在MUTU-1细胞中的典型IC50值分别为0.45 µM和0.47 µM,
-
Organocatalytic Asymmetric Tamura Cycloaddition with α- Branched Nitroolefins: Synthesis of Functionalized 1-Tetralones作者:Utpal Nath、Subhas Chandra PanDOI:10.1021/acs.joc.6b03020日期:2017.3.17A new catalytic asymmetric Tamura cycloaddition with nitroolefins was developed. This demonstration of the reaction of α-branched nitroolefins with homophthalic anhydrides delivers highly functionalized 1-tetralone compounds. With bifunctional squaramide catalyst, the desired tetralone products are obtained with high enantioselectivity and good diastereoselectivity.
-
Immunoassay for phenethylamines of the 2C and DO sub-families申请人:Randox Laboratories Limited公开号:US10775394B2公开(公告)日:2020-09-15Immunoassay methods and their requisite components for the detection and determination of phenethylamines of the 2C and DO sub-families are described.介绍了用于检测和测定 2C 和 DO 亚家族苯乙胺的免疫测定方法及其必要成分。
-
Contemporary state of the investigation of the influence of the discharge of rivers on the hydrologic structure of the Black Sea作者:N. P. Bulgakov、I. Yu. YurkovaDOI:10.1007/bf02519260日期:2000.11
-
In Vivo and in Vitro Studies on the Neurotoxic Potential of 6-Hydroxydopamine Analogs作者:Su Ma、Lorrie Lin、R. Raghavan、Pat Cohenour、Peter Y. T. Lin、Jennifer Bennett、Russell J. Lewis、Eric L. Enwall、Richard KostrzewaDOI:10.1021/jm00020a024日期:1995.9In an attempt to determine which physical and biological properties could best be correlated with neurotoxic potential, seven analogs of 1-(2,4,5-trihydroxyphenyl)-2-aminoethane (1), better known as B-hydroxydopamine, were synthesized and compared to 1 in a variety of ways both in vivo and in vitro. The analogs, in combination with-the standard 1, include all eight of the 2,4,5-trisubstituted-phenyl derivatives of phenethylamine and alpha-methylphenethylamine in which the substitution is of the trihydroxy or aminodihydroxy form. Low (60 nmol) and high (300 nmol) intracerebroventricular doses of all analogs produced long-term (7 day) reduction of mouse whole brain norepinephrine (NE) and lesser depletions of dopamine (DA), and effects on serotonin were varied. The analog 1-(5-amino-2,4-dihydroxyphenyl)-2-aminopropane (8) was both more complete and more selective than the standard 1 in depleting NE. Using a histofluorometric glyoxylic acid method and Fink-Heimer silver degeneration stain, it was determined that overt neural degeneration was produced by 8. In vitro, the ease of oxidation of the eight analogs was found to be represented by a formal potential range of -130 to -212 mV vs SCE. However, there was no obvious relationship between ease of oxidation and the extent of monoamine depletion from mouse brain. Using kinetic analysis of synaptosomal accumulation of [H-3]NE and [H-3]DA, it was found that the standard 1 is more potent in its interaction with the DA uptake site (K-i = 12 +/- 0 mu M) than the NE uptake site (K-i = 51 +/- 1 mu M). A correlation analysis was used to determine that differences in NE and DA depletion by each analog could not be explained by differences in potency for in vitro uptake blockade. However, there was a correlation between the K-i for [H-3]NE uptake blockade and the EC(50) for synaptosomal release of preloaded [H-3]NE for the eight analogs (R(2) = 0.96; for log:log plot, R(2) = 0.54), indicating that the results for these two in vitro tests both reflect interaction with the same NE neuronal membrane transport site. A similar correlation between K-i and EC(50) was shown for all eight analogs using [H-3]DA (R(2) = 0.92; for log:log plot, R(2) = 0.52), indicating interaction with the same DA neuronal membrane transport site. These findings demonstrate that there is no single property that can account for selectivity of action and/or potency of catecholamine neurotoxins related to 6-hydroxydopamine.
表征谱图
-
氢谱1HNMR
-
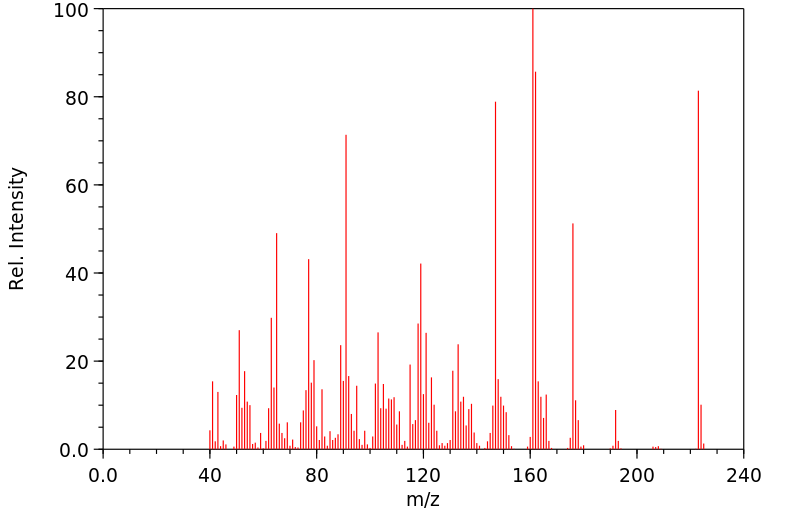
质谱MS
-
碳谱13CNMR
-
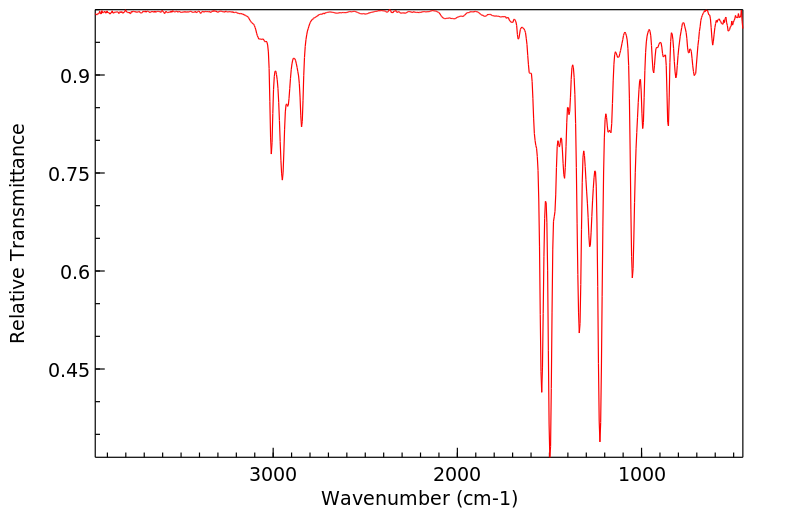
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫