甲基乙烯基硫醚 | 1822-74-8
中文名称
甲基乙烯基硫醚
中文别名
——
英文名称
methyl vinyl sulfide
英文别名
methylenethioethylene;methylsulfanylethene;methyl-vinyl sulfide;Methyl-vinyl-sulfid;(methylthio)-ethene;vinyl methyl sulfide
CAS
1822-74-8
化学式
C3H6S
mdl
——
分子量
74.1466
InChiKey
AMBKPYJJYUKNFI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:69.5°C
-
密度:0.903
-
保留指数:608;608
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:4
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2930909090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:钒(IV)与席夫碱配体的配合物,席夫碱配体来自2,3-二氨基吡啶,可作为催化剂将硫化物氧化为亚砜与H 2 O 2摘要:亚砜是用于合成有价值的配合物和用作药物的物质。在适度的条件下,用冰醋酸作为催化剂,在溶剂的作用下,使用钒(IV)Schiff碱络合物,用(H 2 O 2)过氧化氢将硫化物选择性地氧化为相应的亚砜,生成适当的收率。为了将硫化物转化为各种催化剂的亚砜。必须注意的是,在我们之前的文章中,钒基络合物(VOY 1合成的)用作苯乙烯的环氧化催化剂(Zabardasti和Shangaie,J Iran Chem Soc 13:1875-1886,2016),但在新工作中,钒(IV)与具有2,3的席夫碱配体的配合物使用二氨基吡啶作为催化剂,将硫化物与H 2 O 2氧化为亚砜。据我们所知,没有关于在这些条件下通过钒(IV)Schiff碱与N,O供体配体衍生自2,3-二氨基吡啶催化剂的硫化物选择性氧化为亚砜的文献的描述。选择二甲基硫作为优化实验的图案底物。硫化物的氧化作用在25°C的温度下起作用,其中催化量的钒(IV)络合物或(VOYDOI:10.1007/s13738-018-1480-2
-
作为产物:参考文献:名称:甲基乙烯基硫醚的红外和拉曼光谱、构象稳定性、内旋障碍、振动分配和从头计算摘要:摘要 已经记录了气态和固态甲基-d 3 乙烯基硫化物CH 2 CHSCD 3 的红外和拉曼光谱(3500-40 cm -1 )。此外,还记录了液体的拉曼光谱(3500-80 cm -1 )并获得定性去极化值。流体相光谱与固体光谱的比较清楚地表明,在环境温度下,流体相中存在两种构象异构体。根据气态CH 2 CHSCH 3 的拉曼光谱的温度研究,确定低能量顺式构象异构体和高能量构象异构体之间的焓差为750 ± 68 cm -1 (2.14 ± 0.19 kcal/mol)。气态CH 2 CHSCH 3 和CH 2 CHSCD 3 的远红外光谱已以0的分辨率记录。1 cm -1 和甲基和不对称扭转的基本原理和几个“热带”已被指定。根据这些数据,发现控制不对称扭转的势函数参数(以 cm -1 为单位)为:V 1 = 262 ± 9 (0.75 ± 0.03 kcal/mol),V 2 = 866 ±DOI:10.1016/s0022-2860(97)00266-4
文献信息
-
Nickel Schiff-base complexes immobilized on boehmite nanoparticles and their application in the oxidation of sulfides and oxidative coupling of thiols as novel and reusable nano organometal catalysts作者:Arash Ghorbani-Choghamarani、Bahman Tahmasbi、Fatemeh Arghand、Sara FaryadiDOI:10.1039/c5ra14974f日期:——
Ni-complex-boehmite was prepared in water at room temperature using commercially available materials and applied as an efficient nanocatalyst in the oxidation of sulfides and thiols.
-
Experimental and theoretical studies of the gas-phase protonation of vinyl ethers, vinyl sulfides, and vinyl selenides作者:K. Osapay、J. Delhalle、K. M. Nsunda、E. Rolli、R. Houriet、L. HevesiDOI:10.1021/ja00196a002日期:1989.7Determination des affinites protoniques par mesure des basicites en phase gazeuse dans un spectrometre a resonance cyclotronique ionique et etude theorique par calculs ab initio MO aux niveaux STO-3G et 3-21G*Determination des affinites protoniques par mesure des basicites en phasegazeuse dans un spectrometer a Resonance cyclotronique ionique etude theorique par calculs ab initio MO aux niveaux STO-3G et 3-21G*
-
Difluoro- and trifluoro diazoalkanes – complementary approaches in batch and flow and their application in cycloaddition reactions作者:Katharina J. Hock、Lucas Mertens、Friederike K. Metze、Clemens Schmittmann、Rene M. KoenigsDOI:10.1039/c6gc03187k日期:——Herein we report on applications of fluorinated diazoalkanes in cycloaddition reactions, with the emphasis on studying subtle differences between diverse fluorinated diazo compounds. These differences led to two major synthetic...
-
Selectivity in cycloadditions—-XII作者:P. Caramella、T. Bandiera、P. Grünanger、F. Marinone AlbiniDOI:10.1016/s0040-4020(01)91194-9日期:1984.1Cycloadditions of nitrile oxides to 2,3-dihydrofuran are highly regioselective whereas the regioselectivity of the cycloadditions to 2,3-dihydrothiophen is only moderate. The directing effect of oxygen and sulfur in these cycloadditions could be evaluated at 2.8 and 1.1 Kcal mol-1 respectively. The related acyclic sulfur dipolarophiles, (E)-propenyl methyl and phenyl sulfides, similarly undergo cycloadditions
-
[2 + 2] cycloadditions of 2,2-bis(trifluoromethyl)ethylene-1,1-dicarbonitrile with vinyl sulfides and ketene S,S-acetals作者:Reinhard Brückner、Rolf HuisgenDOI:10.1016/0040-4039(90)80125-6日期:1990.1In its [2+2] cycloadditions to vinyl sulfides, the title olefin (BTF) exceeds tetracyanoethylene up to 8200-fold in rate, but is more sensitive to steric hindrance by β-substituents in the donor olefin; some vinyl sulfides react faster than vinyl ethers with BTF. The dependence of rate on solvent polarity Is In harmony with zwitterionic Intermediates.
表征谱图
-
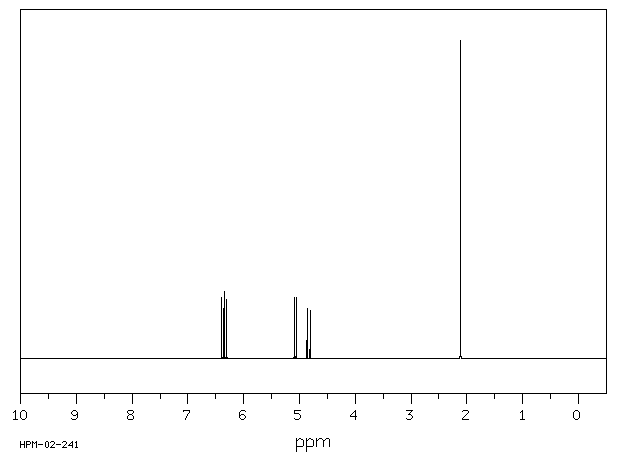
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯