2,3-环氧-2,3-二氢-2-甲基-1,4-萘醌 | 15448-59-6
中文名称
2,3-环氧-2,3-二氢-2-甲基-1,4-萘醌
中文别名
——
英文名称
2,3-epoxy-2-methyl-2,3-dihydro-1,4-naphthoquinone
英文别名
Menadione epoxide;1a-methyl-7aH-naphtho[2,3-b]oxirene-2,7-dione
CAS
15448-59-6
化学式
C11H8O3
mdl
——
分子量
188.183
InChiKey
NNUKDUBCRRYXDC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:100-101 °C
-
沸点:357.4±42.0 °C(Predicted)
-
密度:1.406±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:14
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:46.7
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
反应信息
-
作为反应物:描述:2,3-环氧-2,3-二氢-2-甲基-1,4-萘醌 在 palladium on activated charcoal 氢气 作用下, 以 四氢呋喃 为溶剂, 以80%的产率得到3,4-Dihydroxy-3-methyl-3,4-dihydro-2H-naphthalen-1-one参考文献:名称:Torii, Sigeru; Okumoto, Hiroshi; Nakayasu, Seizo, Chemistry Letters, 1989, p. 1975 - 1978摘要:DOI:
-
作为产物:描述:甲萘醌 在 [Zr6(μ3-O)4(μ3-OH)4(3,3',5,5'-azobenzenetetracarboxylate)2(OH)4(H2O)4] 、 双氧水 作用下, 以 乙腈 为溶剂, 生成 2,3-环氧-2,3-二氢-2-甲基-1,4-萘醌参考文献:名称:Zr基金属有机骨架Zr-abtc和MIP-200在H2O2选择性氧化中的催化性能摘要:在基于H 2 O 2的选择性氧化中研究了由8个连接的Zr 6簇和四位连接体组成的Zr-abtc和MIP-200金属有机骨架的催化性能,并将其与12配位的UiO-66和UiO进行了比较。 ‐67。Zr-abtc在α,β-不饱和酮中缺电子的C = C键的环氧化方面显示了底物转化和产物选择性方面的优势。1,2-环氧化物在香芹酮环氧化中的显着优势,再加上硫醚氧化中砜的高选择性,表明了Zr-abtc上的亲核氧化机理。Zr-abtc中不饱和酮的环氧化中优异的催化性能与大量弱碱性位点有关。H的亲电活化Zr-abtc在石竹烯中富电子的C = C键的环氧化中具有很高的活性,这也可以实现2 O 2。XRD和FTIR研究证实了催化后Zr-abtc结构的保留。MIP-200在基于H 2 O 2的氧化中的低活性很可能与它的特定亲水性有关,这不利于有机底物和H 2 O 2的吸附。DOI:10.1002/chem.202005152
文献信息
-
Activation of H<sub>2</sub>O<sub>2</sub> over Zr(IV). Insights from Model Studies on Zr-Monosubstituted Lindqvist Tungstates作者:Nataliya V. Maksimchuk、Vasilii Yu. Evtushok、Olga V. Zalomaeva、Gennadii M. Maksimov、Irina D. Ivanchikova、Yuriy A. Chesalov、Ilia V. Eltsov、Pavel A. Abramov、Tatyana S. Glazneva、Vadim V. Yanshole、Oxana A. Kholdeeva、R. John Errington、Albert Solé-Daura、Josep M. Poblet、Jorge J. CarbóDOI:10.1021/acscatal.1c02485日期:2021.8.20(Bu4N)2[W5O18Zr(H2O)3] (1) and (Bu4N)6[W5O18Zr(μ-OH)}2] (2), have been employed as molecular models to unravel the mechanism of hydrogen peroxide activation over Zr(IV) sites. Compounds 1 and 2 are hydrolytically stable and catalyze the epoxidation of C═C bonds in unfunctionalized alkenes and α,β-unsaturated ketones, as well as sulfoxidation of thioethers. Monomer 1 is more active than dimer 2. AcidZr-单取代的 Lindqvist 型多金属氧酸盐 (Zr-POM),(Bu 4 N) 2 [W 5 O 18 Zr(H 2 O) 3 ] ( 1 ) 和 (Bu 4 N) 6 [W 5 O 18 Zr( μ-OH)} 2 ] ( 2 ),已被用作分子模型来揭示过氧化氢在 Zr(IV) 位点上的活化机制。化合物1和2具有水解稳定性,可催化未官能化烯烃和 α,β-不饱和酮中 C=C 键的环氧化以及硫醚的磺化氧化。单体1比二聚体2更活跃。酸添加剂大大加速了氧化反应,并将氧化剂利用效率提高到>99%。产物分布表明异裂氧转移机制,该机制涉及在 Zr-POM 和 H 2 O 2相互作用时形成的亲电氧化物质。1和2与 H 2 O 2的相互作用以及由此产生的过氧衍生物已通过 UV-vis、FTIR、拉曼光谱、HR-ESI-MS 和组合 HPLC-ICP-原子发射光谱技术进行了研究。一个之间的相互作用17
-
Chinon-Amin-Reaktionen, 20. Mitt. 1,4-Naphthochinon-Derivate einiger Psychopharmaka mit sekundärer Aminstruktur作者:Hans-Jörg Kallmayer、Christiane TappeDOI:10.1002/ardp.19863190708日期:——Missing.丢失的。
-
PROCESS FOR THE SYNTHESIS OF AMINAPHTONE申请人:LABORATORI BALDACCI SPA公开号:US20140323747A1公开(公告)日:2014-10-30The present invention concerns a new process for the synthesis of aminaphtone, which makes use of non-toxic solvents and reagents, under mild reaction and temperature conditions. The aminaphtone obtained with the method of the present invention also has a purity of at least 98% in weight. The method comprises the following steps: a) epoxidating menadione 1 to provide epoxide 2, b) acidifying epoxide 2 to provide hydroxynaphthoquinone 3, c) esterifying between hydroxynaphthoquinone 3 and 4-aminobenzoyl chloride to obtain compound 4, and d) reducing compound 4 in the presence of a reducing agent in water to obtain aminaphtone.
-
Unprecedented Role of Hydronaphthoquinone Tautomers in Biosynthesis作者:Syed Masood Husain、Michael A. Schätzle、Steffen Lüdeke、Michael MüllerDOI:10.1002/anie.201404560日期:2014.9.8unexpected 1,4‐diketo tautomeric form of 2‐hydroxyhydronaphthoquinone as a stable intermediate. Similar 1,4‐diketo tautomers of hydronaphthoquinones were established as products of the NADPH‐dependent enzymatic reduction of other 1,4‐naphthoquinones, and as substrates for different members of the superfamily of short‐chain dehydrogenases. We propose an essential role of hydroquinone diketo tautomers in
-
Synthesis, Anti-Proliferative Activity Evaluation and 3D-QSAR Study of Naphthoquinone Derivatives as Potential Anti-Colorectal Cancer Agents作者:Julio Acuña、Jhoan Piermattey、Daneiva Caro、Sven Bannwitz、Luis Barrios、Jairo López、Yanet Ocampo、Ricardo Vivas-Reyes、Fabio Aristizábal、Ricardo Gaitán、Klaus Müller、Luis FrancoDOI:10.3390/molecules23010186日期:——active (1.73 < IC50 < 18.11 μM). The naphtho[2,3-b]thiophene-4,9-dione analogs showed potent cytotoxicity, 8-hydroxy-2-(thiophen-2-ylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione being the compound with the highest potency and selectivity. Our results suggest that the toxicity is improved in molecules with tricyclic naphtho[2,3-b]furan-4,9-dione and naphtho[2,3-b]thiophene-4,9-dione systems 2-substituted
表征谱图
-
氢谱1HNMR
-
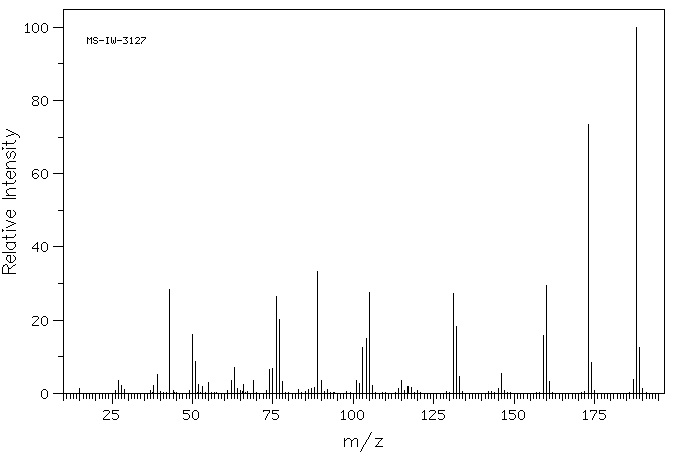
质谱MS
-
碳谱13CNMR
-
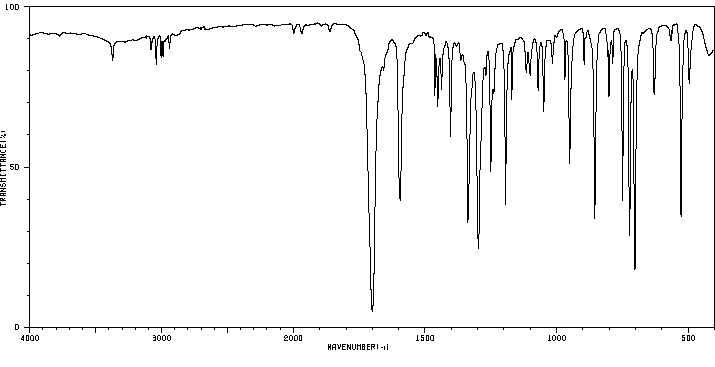
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮