七氟丁酰基乙酸乙酯 | 336-62-9
中文名称
七氟丁酰基乙酸乙酯
中文别名
全氟丁酰基乙酸乙酯
英文名称
ethyl 4,4,5,5,6,6,6-heptafluoro-3-oxohexanoate
英文别名
Ethyl heptafluorobutyrylacetate
CAS
336-62-9
化学式
C8H7F7O3
mdl
MFCD00015326
分子量
284.131
InChiKey
CMGCMFZWEPCGSQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:85°C 40mm
-
密度:1.424±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:18
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:10
安全信息
-
危险等级:FLAMMABLE
-
危险品标志:F,Xi
-
危险类别码:R10,R36/37/38
-
危险品运输编号:UN 1993
-
海关编码:2918300090
-
安全说明:S26,S36
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-Acetyl-4,4,5,5,6,6,6-heptafluoro-3-oxo-hexanoic acid ethyl ester 115479-06-6 C10H9F7O4 326.168 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 2-diazo-4,4,5,5,6,6,6-heptafluoro-3-oxohexanoate 15959-98-5 C8H5F7N2O3 310.128 3,3,4,4,5,5,5-七氟戊-2-酮 heptafluoropropyl methyl ketone 355-17-9 C5H3F7O 212.067 —— Ethyl 4,4,5,5,6,6,6-heptafluoro-3-amino-2-hexanoate 170804-19-0 C8H10F7NO2 285.162
反应信息
-
作为反应物:描述:参考文献:名称:Burdon,J.; McLoughlin,V.C.R., Tetrahedron, 1964, vol. 20, p. 2162 - 2166摘要:DOI:
-
作为产物:描述:2-Acetyl-4,4,5,5,6,6,6-heptafluoro-3-oxo-hexanoic acid ethyl ester 在 氢氧化钾 作用下, 以 乙醇 为溶剂, 以23%的产率得到七氟丁酰基乙酸乙酯参考文献:名称:Hydrolysis of 2-acetyl-substituted fluoroalkyl 3-ketoesters摘要:DOI:10.1007/bf00958359
文献信息
-
[EN] PYRIMIDINE COMPOUND AND ITS USE IN PEST CONTROL<br/>[FR] COMPOSÉ PYRIMIDINE ET SON UTILISATION DANS LA LUTTE ANTIPARASITAIRE
-
Novel diphosphines, their complexes with transisition metals and their use in asymmetric synthesis申请人:——公开号:US20040260101A1公开(公告)日:2004-12-23The invention relates to novel diphosphines, in optically pure or racemic form, of formula (I): 1 in which: R 1 and R 2 are a (C 5 -C 7 )cycloalkyl group, an optionally substituted phenyl group or a 5-membered heteroaryl group; and A is (CH 2 —CH 2 ) or CF 2 . The invention further relates to the use of a compound of formula (I) as a ligand for the preparation of a metal complex useful as a chiral catalyst in asymmetric catalysis, and to the chiral metal catalysts comprising at least one ligand of formula (I).
-
Autocatalyzed three-component cyclization of polyfluoroalkyl-3-oxo esters, methyl ketones and alkyl amines: a novel approach to 3-alkylamino-5-hydroxy-5-polyfluoroalkylcyclohex-2-en-1-ones作者:Marina V. Goryaeva、Svetlana O. Kushch、Olga G. Khudina、Yanina V. Burgart、Yulia S. Kudyakova、Marina A. Ezhikova、Mikhail I. Kodess、Pavel A. Slepukhin、Lilya Sh. Sadretdinova、Natalya P. Evstigneeva、Natalya A. Gerasimova、Victor I. SaloutinDOI:10.1039/c9ob00293f日期:——A new one-pot reaction between polyfluoroalkylated 3-oxo esters, methyl ketones and primary or secondary alkyl amines is reported as an efficient approach to 3-alkylamino-5-hydroxy-5-polyfluoroalkylcyclohex-2-en-1-ones. The scope of three-component cyclization and its plausible mechanism are discussed. The described protocol makes it possible to vary the functional substituents in 2, 3 and 5 positions
-
Biomimetic base-catalyzed [1,3]-proton shift reaction. A practical synthesis of β-fluoroalkyl-β-amino acids作者:Vadim A. Soloshonok、Valery P. KukharDOI:10.1016/0040-4020(96)00300-6日期:1996.5An efficient approach to practical synthesis of β-fluoroalkyl-β-amino acids is described. The method consists in the reducing reagent-free base-catalyzed biomimetic transamination reaction between fluorinated β-keto carboxylic esters and benzylamine. This transformation involves two sequential base-catalyzed [1,3]-proton transfers giving rise to corresponding N-benzylidene derivatives as the products
-
Regiocontrolled N-, O- and C-methylation of 1-phenyl-3-polyfluoroalkyl-1H-pyrazol-5-ols作者:N.A. Nemytova、E.V. Shchegol’kov、Ya.V. Burgart、P.A. Slepukhin、S.S. Borisevich、S.L. Khursan、V.I. SaloutinDOI:10.1016/j.jfluchem.2017.12.011日期:2018.2The approaches for regiocontrolled N-, O- and C-methylation of 1-phenyl-3-polyfluoroalkyl-1H-pyrazol-5-ols have been developed. The chemoselective N-methylation proved to be an efficient method for the synthesis of polyfluorinated antipyrine analogs. In addition, we reinvestigated the structure of 3-polyfluoroalkyl-1-phenylpyrazol-5-ols by the X-Ray analysis. The quantum-chemical calculations were
表征谱图
-
氢谱1HNMR
-
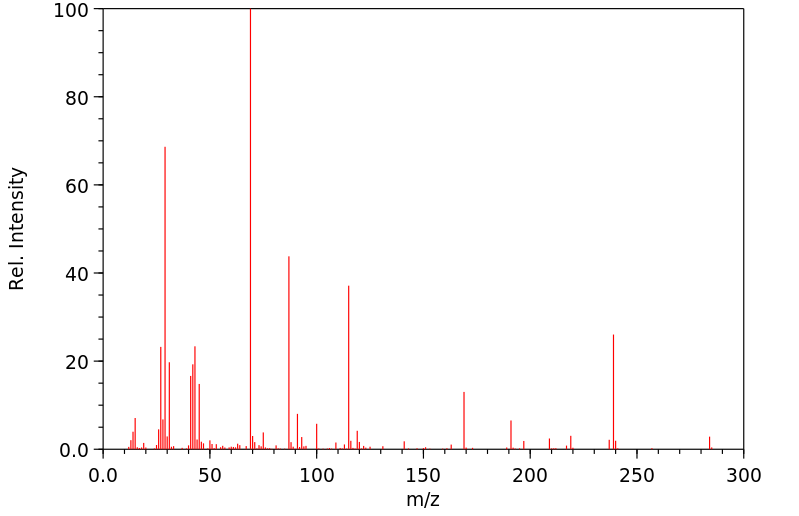
质谱MS
-
碳谱13CNMR
-
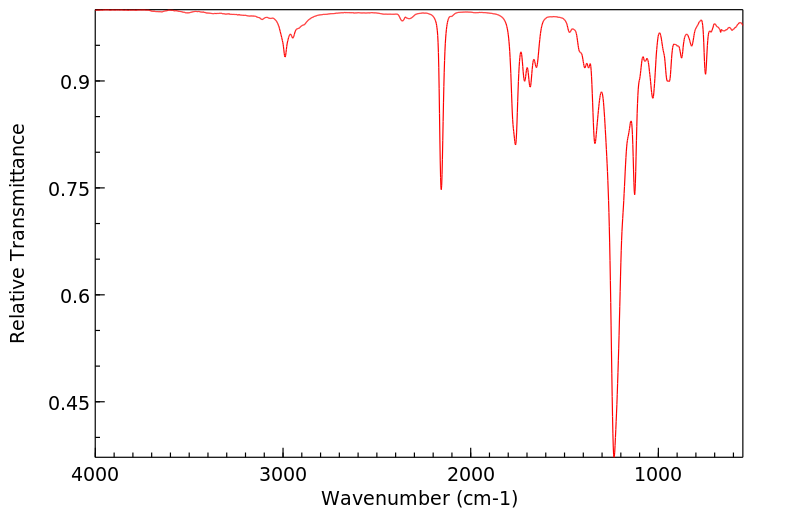
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰基乙酸
顺-3-己烯-1-丙酮酸
青霉酸
钠氟草酰乙酸二乙酯
醚化物
酮霉素
辛酸,2,4-二羰基-,乙基酯
草酸乙酯钠盐
草酰乙酸二乙酯钠盐
草酰乙酸二乙酯
草酰乙酸
草酰丙酸二乙酯
苯乙酰丙二酸二乙酯
苯丁酸,b-羰基-,2-丙烯基酯
聚氧化乙烯
羟基-(3-羟基-2,3-二氧代丙基)-氧代鏻
磷酸二氢2-{(E)-2-[4-(二乙胺基)-2-甲基苯基]乙烯基}-1,3,3-三甲基-3H-吲哚正离子
碘化镝
硬脂酰乙酸乙酯
甲氧基乙酸乙酯
甲氧基乙酰乙酸酯
甲基氧代琥珀酸二甲盐
甲基4-环己基-3-氧代丁酸酯
甲基4-氯-3-氧代戊酸酯
甲基4-氧代癸酸酯
甲基4-氧代月桂酸酯
甲基4-(甲氧基-甲基磷酰)-2,2,4-三甲基-3-氧代戊酸酯
甲基3-羰基-2-丙酰戊酸酯
甲基3-氧代十五烷酸酯
甲基2-氟-3-氧戊酯
甲基2-氟-3-氧代己酸酯
甲基2-氟-3-氧代丁酸酯
甲基2-乙酰基环丙烷羧酸酯
甲基2-乙酰基-4-甲基-4-戊烯酸酯
甲基2-乙酰基-2-丙-2-烯基戊-4-烯酸酯
甲基2,5-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代丁酸酯
甲基1-异丁酰基环戊烷羧酸酯
甲基1-乙酰基环戊烷羧酸酯
甲基1-乙酰基环丙烷羧酸酯
甲基1-乙酰基-2-乙基环丙烷羧酸酯
甲基(2Z,4E,6E)-2-乙酰基-7-(二甲基氨基)-2,4,6-庚三烯酸酯
甲基(2S)-2-甲基-4-氧代戊酸酯
甲基(1S,2R)-2-乙酰基环丙烷羧酸酯
甲基(1R,2R)-2-乙酰基环丙烷羧酸酯
瑞舒伐他汀杂质
瑞舒伐他汀杂质
环氧乙烷基甲基乙酰乙酸酯
环戊戊烯酸,Β-氧代,乙酯