3-甲氧基苯肼盐酸 | 39232-91-2
中文名称
3-甲氧基苯肼盐酸
中文别名
3-甲氧基苯肼盐酸盐
英文名称
m-methoxyphenylhydrazine hydrochloride
英文别名
(3-methoxyphenyl)hydrazine hydrochloride;3-Methoxyphenylhydrazinehydrochloride;hydron;(3-methoxyphenyl)hydrazine;chloride
CAS
39232-91-2
化学式
C7H10N2O*ClH
mdl
MFCD00044416
分子量
174.63
InChiKey
GMXFZBZOVZOYNQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:142°C (dec.)
-
稳定性/保质期:
常规情况下不会分解,也没有任何危险反应。
计算性质
-
辛醇/水分配系数(LogP):1.21
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:47.3
-
氢给体数:3
-
氢受体数:3
安全信息
-
危险等级:6.1
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2928000090
-
危险品运输编号:2811
-
危险类别:6.1
-
包装等级:III
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:密封、阴凉、干燥保存
SDS
| Name: | 3-Methoxyphenylhydrazine hydrochloride 98% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 39232-91-2 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 39232-91-2 | 3-Methoxyphenylhydrazine hydrochloride | 98 | 254-368-4 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Use with adequate ventilation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 39232-91-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin. Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: purple
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 142 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: > 142 deg C
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C7H10N2O.HCl
Molecular Weight: 174.63
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 39232-91-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Methoxyphenylhydrazine hydrochloride - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 39232-91-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 39232-91-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 39232-91-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
3-甲氧基苯肼盐酸属于取代苯肼类有机物,广泛用作医药、农药和染料领域中非常重要的中间体。
制备将0.50摩尔(65.6克)的3-甲氧基苯胺与213克416克18%盐酸溶液(2.0摩尔,213克)混合均匀,降温至0~-5℃。称取0.51摩尔(35克)亚硝酸钠并溶解于82克水中制备30%的水溶液,将此溶液缓慢滴加到上述体系中,1小时后检测无原料剩余,得到重氮盐体系。
将144克(0.87摩尔)亚硫酸钾溶于800克水中,并加入稀盐酸调节pH至6-8之间,降温至0℃。随后将重氮盐体系滴加到亚硫酸钾溶液中,同时滴加15%氢氧化钾水溶液以控制pH在6-8之间。滴加完成后,在0℃下保温1小时,然后将体系加热至60℃反应2小时,检测无重氮盐剩余。
向体系中加入200克(2摩尔,4等当量,36%)的盐酸,升温至100℃反应2小时。体系澄清后进行抽滤,滤液冷却析晶;将滤饼再次抽滤得到湿品130克,含量为63%,最终收率为93.8%。
反应信息
-
作为反应物:参考文献:名称:基于查尔酮的环丙基酮和肼之间的 Cloke-Wilson 型重排串联反应合成四氢哒嗪摘要:开发了一种以环丙基酮和肼为原料合成多取代四氢哒嗪的简便有效的方法。该转化基于查尔酮,通过 HFIP 中 TfOH 催化的 Cloke-Wilson 型重排串联反应发生。DOI:10.1021/acs.joc.3c02824
-
作为产物:参考文献:名称:发现了以FtsZ为目标的含1,3,4-恶二唑-2-酮的苯甲酰胺衍生物,可作为杀死多种MDR细菌菌株的高效药物。摘要:由耐多药(MDR)病原体(例如耐甲氧西林的金黄色葡萄球菌(MRSA)和耐万古霉素的金黄色葡萄球菌(VRSA))引起的感染扩散,引起了人们对具有新作用机制的新型抗生素的需求。细菌分裂蛋白FtsZ已被鉴定为可在临床上开发的新型药物靶标。作为开发靶向FtsZ的抗菌剂的持续努力的一部分,我们在此描述了六种系列的新型含1,3,4-恶二唑-2-one,1,2,4-三唑-的设计,合成和生物活性。含3-one和含吡唑啉-5-one的苯甲酰胺衍生物。其中,化合物A14被发现是最有效的抗菌剂,对所有测试的革兰氏阳性菌株均优于环丙沙星,利奈唑胺和红霉素等临床药物,特别是耐甲氧西林,耐青霉素和临床分离的金黄色葡萄球菌。随后的生物学活性和对接分析研究证明,A14可以作为靶向FtsZ的有效化合物。初步的SAR表明了进一步优化这些新颖类似物的总体方向。综上所述,这项研究为开发新型靶向FtsZ的杀菌剂提供了有希望的化学型。DOI:10.1016/j.bmc.2019.06.010
-
作为试剂:描述:(2S,4R)-1-(3-oxo-thiobutyryl)-4-(2-trifluoromethyl-benzenesulfonyl)-pyrrolidine-2-carboxylic acid methyl ester 、 (2R,4R)-1-(3-oxo-thiobutyryl)-4-(2-trifluoromethyl-benzenesulfonyl)-pyrrolidine-2-carboxylic acid methyl ester 、 3-甲氧基苯肼盐酸 、 (2R,4R)-1-[2-(3-methoxy-phenyl)-5-methyl-2H-pyrazol-3-yl]-4-(2-trifluoromethyl-benzenesulfonyl)-pyrrolidine-2-carboxylic acid methyl ester 在 3-甲氧基苯肼盐酸 作用下, 反应 192.0h, 生成 (2S,4R)-1-[2-(3-Methoxy-phenyl)-5-methyl-2H-pyrazol-3-yl]-4-(2-trifluoromethyl-benzenesulfonyl)-pyrrolidine-2-carboxylic Acid Methyl Ester参考文献:名称:Proline derivatives摘要:本发明涉及一种式为(I)的化合物,其中A,R1-R6在说明书和权利要求书中定义。式(I)化合物可用作药物。公开号:US08163793B2
文献信息
-
N-benzylindol-3-yl propanoic acid derivatives as cyclooxygenase申请人:Merck Frosst Canada, Inc.公开号:US05604253A1公开(公告)日:1997-02-18The invention encompasses the novel compound of Formula I as well as a method of treating cyclooxygenase-2 mediated diseases comprising administration to a patient in need of such treatment of a non-toxic therapeutically effective amount of a compound of Formula I. ##STR1## The invention also encompasses certain pharmaceutical compositions for treatment of cyclooxygenase-2 mediated diseases comprising compounds of Formula I.这项发明涵盖了式I的新化合物,以及一种治疗环氧合酶-2介导疾病的方法,包括向需要此类治疗的患者施用式I化合物的非毒性治疗有效量。 该发明还涵盖了用于治疗环氧合酶-2介导疾病的某些含有式I化合物的药物组合物。
-
Continuous Flow Process For the Synthesis of Phenylhydrazine Salts and Substituted Phenylhydrazine Salts申请人:SHANGHAI HYBRID-CHEM TECHNOLOGIES公开号:US20190152896A1公开(公告)日:2019-05-23The present invention provided a continuous flow process for the synthesis of phenylhydrazine salts and substituted phenylhydrazine salts. Diazotization, reduction, acidic hydrolysis and salifying with acids are innovatively integrated together. Using acidic liquids of aniline or substituted aniline, diazotization reagents, reductants and acids as raw materials, phenylhydrazine derivative salts is obtained through the synthesis process, which is a three-step continuous tandem reaction including diazotization, reduction, acidic hydrolysis and salifying. The described synthesis process is a kind of integrated solutions, which is carried out in an integrated reactor. The feed inlets of the integrated reactor are continuously filled with raw materials. In the integrated reactor, diazotization, reduction, acidic hydrolysis and salifying are carried out continuously and orderly, and phenylhydrazine salts or substituted phenylhydrazine salts is obtained in the outlet of the integrated reactor without interruption. The total reaction time is no more than 20 min.
-
Aerobic Oxidation of Alkyl 2-Phenylhydrazinecarboxylates Catalyzed by CuCl and DMAP作者:Min Hye Kim、Jinho KimDOI:10.1021/acs.joc.7b03119日期:2018.2.2various fruitful organic reactions such as a catalytic Mitsunobu reaction were reported by virtue of alkyl 2-phenylazocarboxylates, however, the synthesis of alkyl 2-phenylazocarboxylates largely depended on the stoichiometric use of toxic oxidants. In this manuscript, an environment-friendly aerobic oxidative transformation of alkyl 2-phenylhydrazinecarboxylates to alkyl 2-phenylazocarboxylates is
-
[EN] DIHYDROPYRIDAZINONES SUBSTITUTED WITH PHENYLUREAS<br/>[FR] DIHYDROPYRIDAZINONES SUBSTITUÉES PAR DES PHÉNYLURÉES申请人:BAYER PHARMA AG公开号:WO2018086703A1公开(公告)日:2018-05-17Compounds of formula (I) which are nicotinamide phosphoribosyltransferase (NAMPT) inhibitors and their use for the treatment of hyperproloferative diseases and/or disorders responsive to induction of cell death.
-
[EN] PYRAZOLOPYRIMIDINES AS KINASE INHIBITORS<br/>[FR] INHIBITEURS DE KINASE SOUS FORME DE PYRAZOLOPYRIMIDINES
表征谱图
-
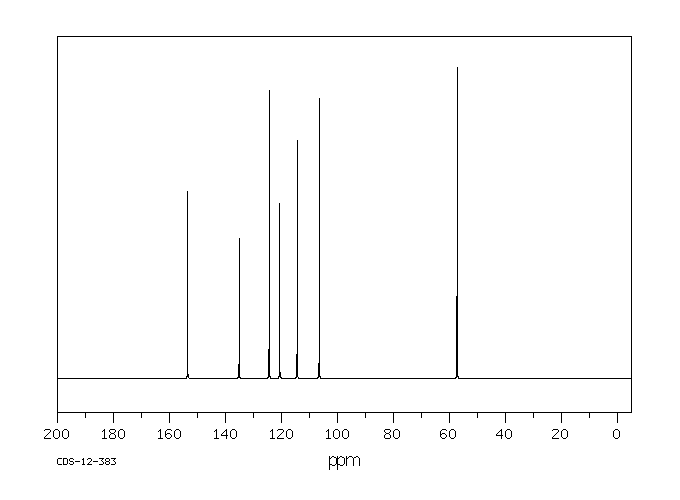
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
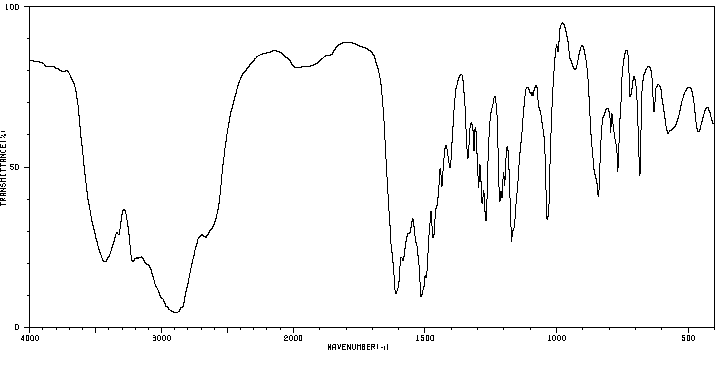
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫