L-脯氨酸,1-乙酰基-4-[[(4-甲基苯基)磺酰]氧代]-,反- | 100895-61-2
中文名称
L-脯氨酸,1-乙酰基-4-[[(4-甲基苯基)磺酰]氧代]-,反-
中文别名
——
英文名称
N-acetyl-trans-4-(tosyloxy)-L-proline
英文别名
trans-1-acetyl-4-(toluene-4-sulfonyloxy)-L-proline;trans-1-Acetyl-4-(toluol-4-sulfonyloxy)-L-prolin;(2S,4R)-1-acetyl-4-(4-methylphenyl)sulfonyloxypyrrolidine-2-carboxylic acid
CAS
100895-61-2
化学式
C14H17NO6S
mdl
——
分子量
327.358
InChiKey
ZGILGOQVJWEWSV-YPMHNXCESA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:579.6±50.0 °C(Predicted)
-
密度:1.44±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:22
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.43
-
拓扑面积:109
-
氢给体数:1
-
氢受体数:6
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (3R,5S)-5-(methoxycarbonyl)-1-acetylpyrrolidin-3-yl 4-methylbenzenesulfonate 57750-51-3 C15H19NO6S 341.385 N-乙酰-L-4-羟基脯氨酸 oxaceprol 33996-33-7 C7H11NO4 173.169 —— methyl 1-acetyl-trans-4-hydroxy-L-proplinate 67943-19-5 C8H13NO4 187.196 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-O-tert-butyl 2-O-methyl (2S,5S)-5-[[(2S,4R)-1-acetyl-4-(4-methylphenyl)sulfonyloxypyrrolidine-2-carbonyl]sulfanylmethyl]pyrrolidine-1,2-dicarboxylate 161424-47-1 C26H36N2O9S2 584.712 —— cis-1-acetyl-4-hydroxy-DL-proline 33996-33-7 C7H11NO4 173.169
反应信息
-
作为反应物:参考文献:名称:Neuberger, Journal of the Chemical Society, 1945, p. 431摘要:DOI:
-
作为产物:描述:参考文献:名称:Neuberger, Journal of the Chemical Society, 1945, p. 431摘要:DOI:
文献信息
-
Studies on Hydroxyproline作者:Arthur A. Patchett、Bernhard WitkopDOI:10.1021/ja01558a050日期:1957.1
-
Studies of N-terminal templates for .alpha.-helix formation. Synthesis and conformational analysis of (2S,5S,8S,11S)-1-acetyl-1,4-diaza-3-keto-5-carboxy-10-thiatricyclo[2.8.1.04,8]tridecane (Ac-Hel1-OH)作者:D. S. Kemp、Timothy P. Curran、William M. Davis、James G. Boyd、Christopher MuendelDOI:10.1021/jo00023a037日期:1991.11A convergent synthesis of the title compound, a conformationally restricted analogue of acetyl-L-prolyl-L-proline, from 1-acetyl-2(S)-carboxy-4(S)-mercaptopyrrolidine and trans-1-(tert-butoxycarbonyl)-2(S)-(methoxy-carbonyl)-5(S)-[(tosyloxy)methyl]pyrrolidine (Ac-Hel1-OH) is reported, along with a synthesis of (2S,8S,11S)-1-acetyl-1,4-diaza-3-keto-10-thiatricyclo[2.8.0(4,8)]tridecane. In the crystal and in CDCl3 solution the conformation of Ac-Hel1-OH is shown to approximate a staggered orientation at the 8,9-CC bond and an s-cis orientation at the acetyl amide bond. In DMF, DMSO, MeCN, and D2O significant amounts of the s-trans conformer are also present.
-
(2S,5S,8S,11S)-1-Acetyl-1,4-diaza-3-keto-5-carboxy-10-thia-tricyclo-[2.8.04,8]-tridecane, 1 synthesis of prolyl-proline-derived, peptide-functionalized templates for α-helix formation作者:D.S. Kemp、Timothy P. CurranDOI:10.1016/s0040-4039(00)80644-9日期:1988.1
-
An Improved Synthesis of a Template for .alpha.-Helix Formation作者:Kim F. McClure、Peter Renold、D. S. KempDOI:10.1021/jo00107a028日期:1995.1
-
KEMP, D. S.;CURRAN, TIMOTHY P., TETRAHEDRON LETT., 29,(1988) N 39, C. 4931-4934作者:KEMP, D. S.、CURRAN, TIMOTHY P.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
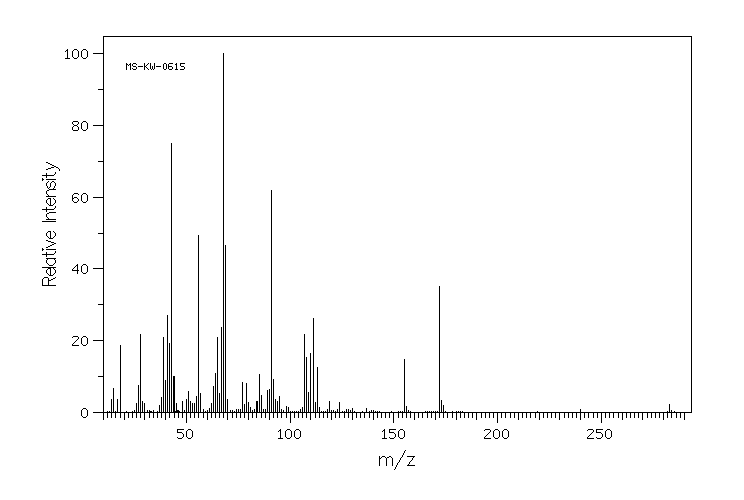
质谱MS
-
碳谱13CNMR
-
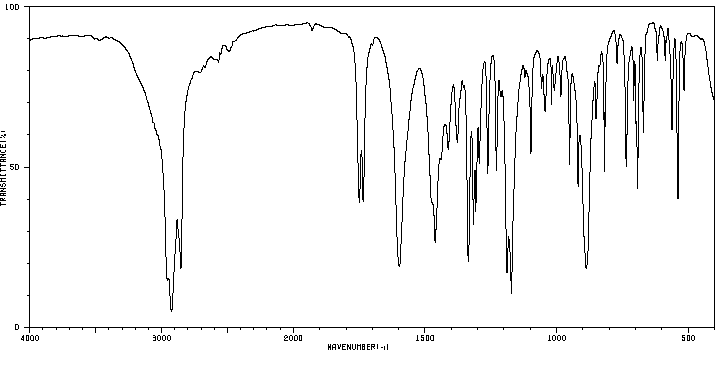
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸