(4-氯苯基)乙烯磺酸盐 | 3058-82-0
中文名称
(4-氯苯基)乙烯磺酸盐
中文别名
——
英文名称
4-chlorophenyl ethenesulfonate
英文别名
ethenesulfonic acid-(4-chloro-phenyl ester);Aethensulfonsaeure-(4-chlor-phenylester);Vinylsulfonsaeure-<4-chlorphenylester>;Vinylsulfonsaeure-p-chlorphenylester;Ethenesulfonic acid, p-chlorophenyl ester;(4-chlorophenyl) ethenesulfonate
CAS
3058-82-0
化学式
C8H7ClO3S
mdl
MFCD00806567
分子量
218.661
InChiKey
IBSQGRPUEMBWMQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:160-163 °C(Press: 11 Torr)
-
密度:1.3970 g/cm3
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2906299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙烯磺酸苯酯 phenyl vinylsulfonate 1562-34-1 C8H8O3S 184.216
反应信息
-
作为反应物:描述:(4-氯苯基)乙烯磺酸盐 、 2-氯-1.3-丁二烯 以 various solvent(s) 为溶剂, 生成 4-Chlor-cyclohex-3-en-1-sulfonsaeure-(4-chlorphenylester)参考文献:名称:Lonchambon,G., Comptes Rendus des Seances de l'Academie des Sciences, Serie C: Sciences Chimiques, 1971, vol. 273, p. 510 - 513摘要:DOI:
-
作为产物:描述:参考文献:名称:Esajan et al., Izvestiya Akademii Nauk Armyanskoi SSR, Khimicheskie Nauki, 1959, vol. 12, p. 221,222摘要:DOI:
文献信息
-
Unique Reactivity of α-Substituted Electron-Deficient Allenes using Sulfinate Salts as Lewis Base Organocatalysts作者:Thomas Martzel、Jean-François Lohier、Annie-Claude Gaumont、Jean-François Brière、Stéphane PerrioDOI:10.1002/adsc.201600929日期:2017.1.4efficient sulfinate‐catalyzed intermolecular addition reaction of α‐substituted allenyl sulfones and allenoates with Michael acceptors is highlighted. The sequence proceeds under mild conditions to provide a scalable and efficient access to versatile functionalized alkynes, displaying a quaternary stereocentre at the propargylic position. This work enriches the diversity of Lewis base organocatalysts
-
Novel Hybrid Conjugates with Dual Suppression of Estrogenic and Inflammatory Activities Display Significantly Improved Potency against Breast Cancer作者:Wentao Ning、Zhiye Hu、Chu Tang、Lu Yang、Silong Zhang、Chune Dong、Jian Huang、Hai-Bing ZhouDOI:10.1021/acs.jmedchem.8b00224日期:2018.9.27of them showed better antiproliferative efficacy in MCF-7 cell lines with IC50 up to 3.7 μM. In vivo experiments in a MCF-7 breast cancer model in Balb/c nude mice indicated that compound 26a was more potent than tamoxifen. Exploration of the compliancy of the structure against ER specificity utilizing these types of isomeric three-dimensional ligands indicated that one enantiomer had much better biological在这项工作中,我们通过将已知的NF-κB抑制剂白藜芦醇(RES)掺入到小鼠体内,开发了一个具有双重抑制活性的新型OBHS-RES杂合化合物的小型文库,该化合物具有针对雌激素受体α(ERα)和NF-κB的双重抑制活性。雌激素受体(ER)的特权间接拮抗结构基序(OBHS,氧杂环庚烯磺酸盐)。OBHS-RES偶联物可以很好地与ER结合,并表现出显着的ERα拮抗活性,并且在巨噬细胞RAW 264.7细胞中也表现出出色的NO抑制作用。用4-羟基他相比,它们中的一些显示在MCF-7细胞系IC更好的抗增殖功效50达3.7μM。在Balb / c裸鼠的MCF-7乳腺癌模型中进行的体内实验表明,化合物26a比他莫昔芬更有效。利用这些类型的异构体三维配体探索结构对内质网特异性的顺应性表明,一种对映异构体具有比另一种对映异构体更好的生物学活性。
-
Development of Selective Estrogen Receptor Modulator (SERM)-Like Activity Through an Indirect Mechanism of Estrogen Receptor Antagonism: Defining the Binding Mode of 7-Oxabicyclo[2.2.1]hept-5-ene Scaffold Core Ligands作者:Yangfan Zheng、Manghong Zhu、Sathish Srinivasan、Jerome C. Nwachukwu、Valerie Cavett、Jian Min、Kathryn E. Carlson、Pengcheng Wang、Chune Dong、John A. Katzenellenbogen、Kendall W. Nettles、Hai-Bing ZhouDOI:10.1002/cmdc.201200048日期:2012.6we discovered estrogen receptor (ER) ligands with a novel three‐dimensional oxabicyclo[2.2.1]heptene core scaffold and good ER binding affinity act as partial agonists via small alkyl ester substitutions on the bicyclic core that indirectly modulate the critical switch helix in the ER ligand binding domain, helix 12, by interactions with helix 11. This contrasts with the mechanism of action of tamoxifen以前,我们发现雌激素受体 (ER) 配体具有新型三维氧杂双环 [2.2.1] 庚烯核心支架和良好的 ER 结合亲和力,通过双环核心上的小烷基酯取代间接调节关键开关螺旋作为部分激动剂在 ER 配体结合域中,螺旋 12,通过与螺旋 11 相互作用。这与他莫昔芬的作用机制形成对比,他莫昔芬直接将螺旋 12 推出基因激活所需的构象。我们现在报告在双环核心支架的这个位置可以容忍更大的取代,即苯磺酸基团,它定义了雌激素受体的新结合表位。我们制备了一系列 14 种氧杂双环庚烯磺酸盐,不同的苯基磺酸盐基团。与母体化合物一样,5,邻位取代基对 ERα 的亲和力最高,对位取代基对 ERα 的亲和力最低。一些类似物表现出与 OBHS 本身相当的 ERα 结合亲和力,或者在邻氯类似物的情况下,高于 OBHS 本身。在基于细胞的研究中,我们发现了几种化合物的活性特征与他莫昔芬相当,但完全作为间接拮抗剂,变构干扰了辅激活蛋白向受体的募集。因此,OBHS
-
一种含有白藜芦醇基团的氧桥双环庚烯类化 合物及其制备和使用方法申请人:苏州楚凯药业有限公司公开号:CN107188896B公开(公告)日:2020-04-03
-
一类含有苯硒基团的选择性雌激素受体调节 剂类化合物及其在抗乳腺癌药物中的应用
表征谱图
-
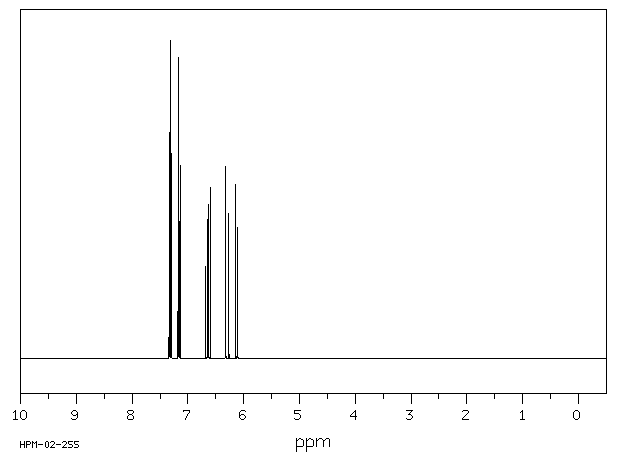
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
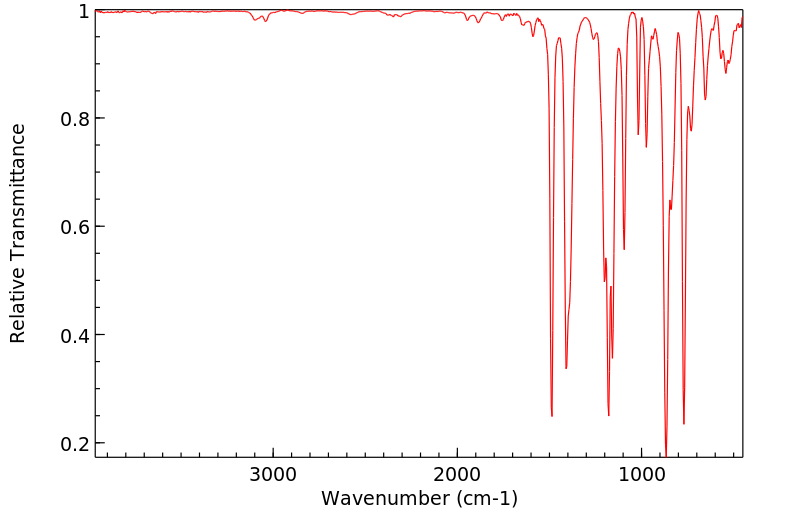
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫