5-甲基-2-己酮肟 | 624-44-2
中文名称
5-甲基-2-己酮肟
中文别名
5-甲-2-己酮肟;甲基异戊基酮肟
英文名称
Methyl-isoamyl-ketoxim
英文别名
Methyl-isoamyl-keton-oxim;5-methyl-hexan-2-one oxime;2-Methyl-hexanoxim-(5);5-Methyl-hexan-2-on-oxim;N-(5-methylhexan-2-ylidene)hydroxylamine
CAS
624-44-2
化学式
C7H15NO
mdl
MFCD00013934
分子量
129.202
InChiKey
ZVZUNLVAVHYXNI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:239.29°C (rough estimate)
-
密度:0.89
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.857
-
拓扑面积:32.6
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2928000090
-
储存条件:常温常压下保存。
SDS
5-甲基-2-己酮肟 修改号码:5
模块 1. 化学品
产品名称: 5-Methyl-2-hexanone Oxime
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5-甲基-2-己酮肟
百分比: >97.0%(GC)
CAS编码: 624-44-2
俗名: Isoamyl Methyl Ketoxime
分子式: C7H15NO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
5-甲基-2-己酮肟 修改号码:5
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.89
溶解度:
[水] 无资料
[其他溶剂] 无资料
5-甲基-2-己酮肟 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ipr-mus LD50:100 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: MP4025000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
5-甲基-2-己酮肟 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 5-Methyl-2-hexanone Oxime
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5-甲基-2-己酮肟
百分比: >97.0%(GC)
CAS编码: 624-44-2
俗名: Isoamyl Methyl Ketoxime
分子式: C7H15NO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
5-甲基-2-己酮肟 修改号码:5
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.89
溶解度:
[水] 无资料
[其他溶剂] 无资料
5-甲基-2-己酮肟 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ipr-mus LD50:100 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: MP4025000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
5-甲基-2-己酮肟 修改号码:5
模块16 - 其他信息
N/A
反应信息
-
作为反应物:参考文献:名称:亚甲基自由基触发的分子间远端C(sp 3)–H通过1,5-氢原子转移(HAT)级联的杂芳基化作用摘要:通过亚胺基引发的1,5-氢原子转移(HAT)级联反应,开发了一种有效的铁催化的烷基酮的分子间远程C(sp 3)-H杂芳基化反应。该方案适用于多种烷基酮和杂芳基,因此为烷基酮和杂芳基的后期官能化提供了一种直接的方法。DOI:10.1021/acs.orglett.8b03865
-
作为产物:描述:magnesium,2-methylbutane,bromide 在 吡啶 、 copper(l) iodide 、 盐酸羟胺 作用下, 以 四氢呋喃 、 甲醇 为溶剂, 反应 9.0h, 生成 5-甲基-2-己酮肟参考文献:名称:叔碳自由基的羰基化:内酰胺的合成†摘要:在此,我们公开了一种有趣的铁催化的叔碳自由基羰基化方法。由1,5-氢原子转移产生的叔碳自由基可以被CO气体平稳地捕获。化学高选择性地构建了多种六元内酰胺。DOI:10.1039/c9cc02112d
文献信息
-
Photoinduced, Copper-Catalyzed Three-Component Annulation of <i>gem</i>-Dialkylthio Enynes作者:Jiang Lou、Juan Ma、Bao-Hua Xu、Yong-Gui Zhou、Zhengkun YuDOI:10.1021/acs.orglett.0c01645日期:2020.7.2Photoinduced, copper-catalyzed three-component radical annulation of gem-dialkylthio enynes, cyclobutanone oxime esters, and boronic acids was achieved, forming highly functionalized aryl thienyl sulfides with both good chemo- and diastereoselectivities. The reaction proceeds through a domino sequence involving cyanoalkyl radical-mediated intramolecular annulation of gem-dialkylthio enyne, alkenyl
-
Iminyl Radical-Mediated Controlled Hydroxyalkylation of Remote C(<i>sp</i> <sup>3</sup> )-H Bond via Tandem 1,5-HAT and Difunctionalization of Aryl Alkenes作者:Zhi-Yong Ma、Li-Na Guo、Yu-Rui Gu、Li Chen、Xin-Hua DuanDOI:10.1002/adsc.201801198日期:2018.11.16A visible‐light mediated γ‐hydroxyalkylation of ketones via C(sp3)‐H functionalization has been developed under redox neutral conditions. This protocol relies on the iminyl radical‐triggered 1,5‐HAT followed by oxyalkylation of alkenes, wherein C−C and C−O bonds were constructed in one step. This three‐component reaction features mild conditions, wide substrate scope and excellent functional group在氧化还原中性条件下,通过C(sp 3)-H官能团形成了可见光介导的酮的γ-羟烷基化反应。该协议依赖于亚胺基引发的1,5-HAT,然后进行烯烃的氧烷基化,其中CC和C-O键是一步构建的。该三组分反应具有温和的条件,宽的底物范围和出色的官能团耐受性,因此可以轻松高效地获得复杂的有价值的酮。
-
<i>O</i>-Perfluoropyridin-4-yl Oximes: Iminyl Radical Precursors for Photo- or Thermal-Induced N–O Cleavage in C(sp<sup>2</sup>)–C(sp<sup>3</sup>) Bond Formation作者:Peng-Ju Xia、Yuan-Zhuo Hu、Zhi-Peng Ye、Xu-Jie Li、Hao-Yue Xiang、Hua YangDOI:10.1021/acs.joc.9b03251日期:2020.3.6group was first installed onto cycloketone oximes as a new electrophore, which was proven to be efficient iminyl radical precursors under photocatalytic and thermal conditions. A range of O-perfluoropyridin-4-yl oximes were successfully utilized in C(sp2)-C(sp3) bond formations of quinoxalin-2(1H)-ones and alkenes, providing facile accesses to a range of functionalized alkylnitriles.
-
A Rapid, Efficient Method for Deprotection of Oximes to Carbonyl Compounds with NaClO<sub>2</sub> in Water作者:Yuan-Yuan Hao、Xian-Liang ZhaoDOI:10.2174/157017861201150112125024日期:2015.1.1A rapid, efficient method for deprotection of oximes to carbonyl compounds is demonstrated by using sodium chlorite (NaClO2) in water. The protocol has been found to be applicable to a wide range of aldoximes and ketoximes with good to excellent yields of the corresponding carbonyl compounds.
-
一种含氟肟醚的合成方法及其应用
表征谱图
-
氢谱1HNMR
-
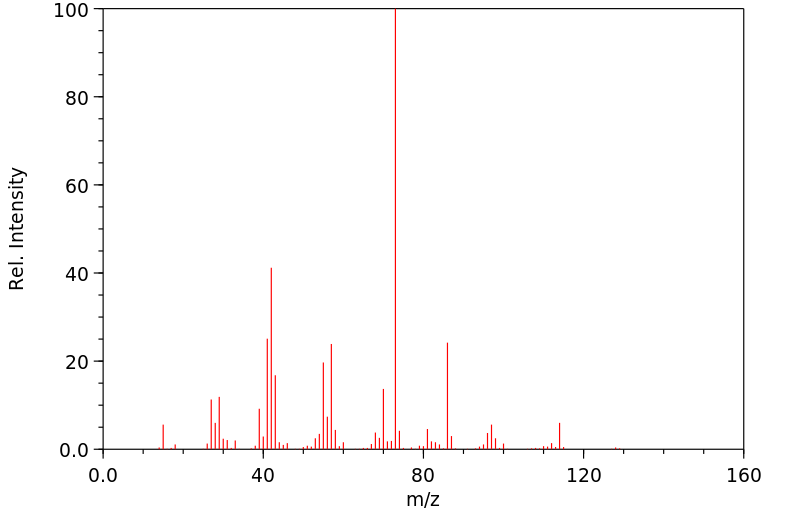
质谱MS
-
碳谱13CNMR
-
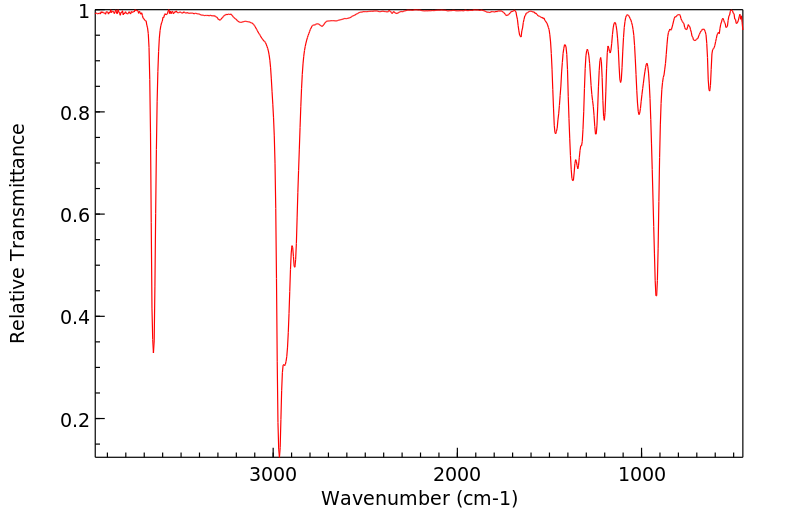
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷