胍基丙酸 | 353-09-3
中文名称
胍基丙酸
中文别名
beta-丙酸胍;3-胍基丙酸
英文名称
3-guanidinopropionic acid
英文别名
β-guanidinopropionic acid;β-GPA;3-Guanidinopropionate;3-(diaminomethylideneazaniumyl)propanoate
CAS
353-09-3
化学式
C4H9N3O2
mdl
MFCD00045939
分子量
131.134
InChiKey
KMXXSJLYVJEBHI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:222 °C (dec.)(lit.)
-
沸点:299.1±42.0 °C(Predicted)
-
密度:1.46±0.1 g/cm3(Predicted)
-
溶解度:酸水溶液(微溶),水(微溶,加热)
-
LogP:-1.680 (est)
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):-1.8
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:102
-
氢给体数:3
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2925290090
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:AY3157500
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:-20°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 3-Guanidinopropionic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3-Guanidinopropionic acid
CAS number: 353-09-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C4H9N3O2
Molecular weight: 131.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 3-Guanidinopropionic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3-Guanidinopropionic acid
CAS number: 353-09-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C4H9N3O2
Molecular weight: 131.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
背景及概述
胍类化合物自古以来广泛应用于医药、农药和化工领域,包括作为杀菌消毒剂、催化剂、营养剂、饲料以及化妆品添加剂。常用的有肌酸及其衍生物、胍基乙酸、胍基丙酸等这些2-3个碳原子的胍基类化合物。胍基丙酸是一种白色晶体,溶于水,是氨基酸衍生物的一种,也是重要的化工原料和中间体。它存在于哺乳动物的血清、脑部、肝脏、肾脏和尿液中。
研究发现,胍基丙酸可以提高胰岛素敏感性并选择性降低脂肪组织重量,有助于治疗和预防新陈代谢紊乱、高血糖、高胰岛素抵抗、高血脂等症状,并且被明确为尿毒症毒素。在正常人的血液中浓度较低,约为0.02μmol/100 mL,在肾衰患者血液中的浓度显著增高,尤其是在低蛋白饮食的尿毒症患者的血液中可以达到约 0.70μmol/100 mL。
胍基丙酸是胍基复合物中唯一能够同时降低血液还原性谷胱甘肽浓度并使G-6-PD活性下降的物质。其在尿毒症血浆中的含量最高,与BUN相关,且能显著抑制中性粒细胞代谢,对机体免疫存在潜在危害。
合成路线 方法1以硫脲β-丙氨酸为原料通过亲核取代胺的烷基化两步反应制备胍基丙酸。该方法含量大于99.0%,总收率76.2%,成本低、条件温和、操作简单,适合工业化生产。
具体反应过程如下:
方法2- 在反应釜中加入异丙醇、3-氨基丙酸和10%氢氧化钠溶液,室温搅拌溶解。
- 向碱性混合溶液滴加单氰胺溶液,在一定温度下搅拌回流一段时间。
- 反应完毕后减压浓缩,蒸出溶剂,得到胍基丙酸粗品。
- 投入胍基丙酸粗品、水和异丙醇于反应釜中常温搅拌一段时间后抽滤、漂洗、烘干,最终得到胍基丙酸。
一种3-胍基丙酸制备方法包括以下步骤:
- 缩合反应:在特定条件下合成化合物。
- 分离与纯化:通过离子交换和醇析结晶等过程进一步提纯。
胍基丙酸主要用于有机合成、降低血压原料药的主要中间体以及食品添加剂、化妆品表面活性剂、饲料添加剂和营养强化剂等领域。
主要参考资料- 潘海龙等(2015). 胍基丙酸的合成.《浙江化工》, 46(7):23-25.
- 郭礼强等(申请号 CN201310275611.2,申请日2013-07-03). 一种3-胍基丙酸的制备方法.
- 杨波等(2014). 苯磺酸左旋氨氯地平对胍基丙酸加重残余肾功能损害的干预研究.《中国现代医学杂志》, 24 (24):15-20.
- 周彬(申请号 CN201110210823.3,申请日2012-01-25). 胍基丙酸的化学合成方法.
主要用于食品、饮料、饲料添加剂、营养强化剂、化妆品用表面活性剂和医药原料等领域。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-guanidinopropionaldehyde 1221685-79-5 C4H9N3O 115.135
反应信息
-
作为反应物:参考文献:名称:Pharmaceutically acceptable salts of B-guanidinopropionic acid with improved properties and uses thereof摘要:本发明涉及β-GPA的新药物盐,具有改善的物理特性。具体来说,本发明涉及具有改善流动性能(例如,改善的卡尔指数和/或豪斯纳比)的β-GPA盐,例如富马酸盐、琥珀酸盐和草酸盐。本发明还涉及包括一种或多种β-GPA盐的药物组合物,以及治疗癌症的方法,包括将包括本发明的β-GPA盐的制剂用于需要的受试者的给药。公开号:US09884813B1
-
作为产物:描述:3-guanidinopropionaldehyde 在 Pisum sativum aminoaldehyde dehydrogenase 2 、 nicotinamide adenine dinucleotide 作用下, 生成 胍基丙酸参考文献:名称:Structural and Functional Characterization of Plant Aminoaldehyde Dehydrogenase from Pisum sativum with a Broad Specificity for Natural and Synthetic Aminoaldehydes摘要:Aminoaldehyde dehydrogenases (AMADHs, EC 1.2.1.19) belong to the large aldehyde dehydrogenase (ALDH) superfamily, namely, the ALDH9 family. They oxidize polyamine-derived omega-aminoaldehydes to the corresponding omega-amino acids. Here, we report the first X-ray structures of plant AMADHs: two isoenzymes, PsAMADH1 and PsAMADH2, from Pisum sativum in complex with beta-nicotinamide adenine dinucleotide (NAD(+)) at 2.4 and 2.15 angstrom resolution, respectively. Both recombinant, proteins are dimeric and, similarly to other ALDHs, each monomer is composed of an oligomerization domain, a coenzyme binding domain and a catalytic domain. Each subunit binds NAD(+) as a coenzyme, contains a solvent-accessible C-terminal peroxisomal targeting signal (type 1) and a cation bound in the cavity close to the NAD(+) binding site. While the NAD(+) binding mode is classical for PsAMADH2, that for PsAMADH1 is unusual among ALDHs. A glycerol molecule occupies the substrate binding site and mimics a bound substrate. Structural analysis and substrate specificity study of both isoenzymes in combination with data published previously on other ALDH9 family members show that the established categorization of such enzymes into distinct groups based on substrate specificity is no more appropriate, because many of them seem capable of oxidizing a large spectrum of aminoaldehyde substrates. PsAMADH1 and PsAMADH2 can oxidize N,N,N-trimethyl-4-aminobutyraldehyde into gamma-butyrobetaine, which is the carnitine precursor in animal cells. This activity highly suggests that in addition to their contribution to the formation of compatible osmolytes such as glycine betaine, beta-alanine betaine and gamma-aminobutyric acid, AMADHs might participate in carnitine biosynthesis in plants. (C) 2009 Elsevier Ltd. All rights reserved.DOI:10.1016/j.jmb.2009.12.015
文献信息
-
[EN] INHIBITORS OF CREATINE TRANSPORT AND USES THEREOF<br/>[FR] INHIBITEURS DE TRANSPORT DE CRÉATINE ET LEURS UTILISATIONS申请人:RGENIX INC公开号:WO2016176636A1公开(公告)日:2016-11-03This invention relates to compounds that inhibit creatine transport and/or creatine kinase, pharmaceutical compositions including such compounds, and methods of utilizing such compounds and compositions for the treatment of cancer.
-
Preparation of N-Formamidinylamino Acids from Amino and Formamidinesulfinic Acids
-
[EN] INHIBITORS OF CREATINE TRANSPORT AND USES THEREOF<br/>[FR] INHIBITEURS DE TRANSPORT DE LA CRÉATINE ET LEURS UTILISATIONS申请人:RGENIX INC公开号:WO2015168465A1公开(公告)日:2015-11-05This invention relates to compounds that inhibit creatine transport and/or creatine kinase, pharmaceutical compositions including such compounds, and methods of utilizing such compounds and compositions for the treatment of cancer.
-
A Facile Conversion of Amino Acids to Guanidino Acids作者:Audrey E. Miller、Judith J. BischoffDOI:10.1055/s-1986-31777日期:——The conversion of amino acids to guanidino acids by the action of aminoiminomethanesulfonic acids (2a-c) is reported. Compounds 2a-c were synthesized by peracetic acid oxidation of the corresponding thioureas.
-
Amidination of Amines under Microwave Conditions Using Recyclable Polymer-Bound 1<i>H</i>-Pyrazole-1-carboxamidine作者:Andreas Kirschning、Wladimir Solodenko、Patrick Bröker、Josef Messinger、Uwe SchönDOI:10.1055/s-2006-926284日期:——A convenient one-step transformation of primary and secondary amines into the corresponding unprotected guanidines using 4-benzyl-3,5-dimethyl-1H-pyrazole-1-carboxamidine and its polymer-bound variant is described. The scopes and limitations of the method, the microwave-assistance of amidination as well as a recycling protocol are examined.报道了一种便捷的单步转化方法,可以将一级和二级胺转化为相应的无保护基胍化合物,所用试剂为4-苄基-3,5-二甲基-1H-吡唑-1-羰基氨脒及其聚合物衍生试剂。本文考察了该方法的应用范围和局限性、微波辅助酰胺化反应以及回收协议。
表征谱图
-
氢谱1HNMR
-
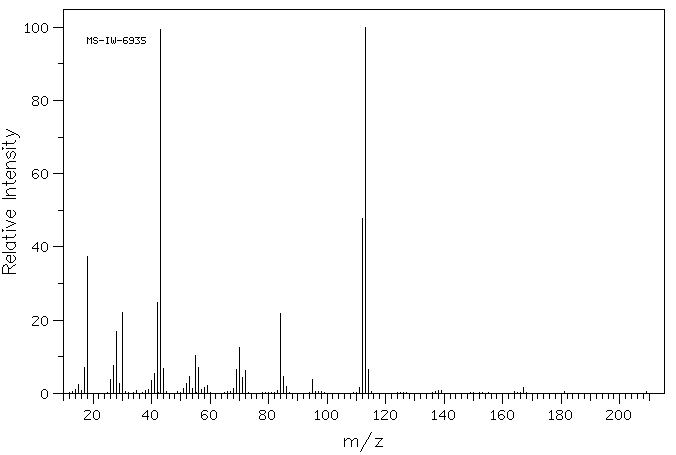
质谱MS
-
碳谱13CNMR
-
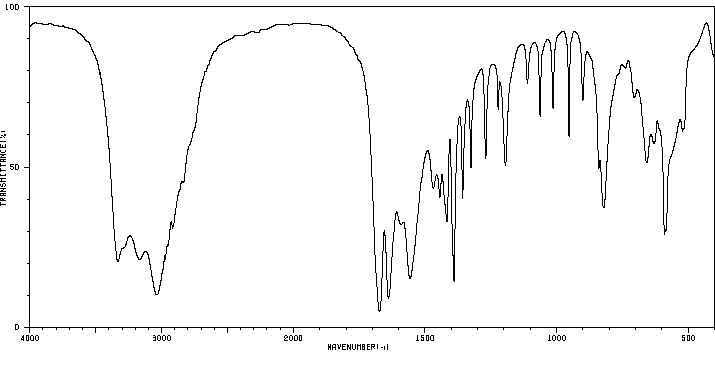
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷