3-羧基异硫氰酸苯酯 | 2131-63-7
中文名称
3-羧基异硫氰酸苯酯
中文别名
3-羧基苯基异硫氰酸酯;3-异硫代氰酰基苯甲酸
英文名称
3-isothiocyanatobenzoic acid
英文别名
3-Isothiocyanato-benzoesaeure;3-Carboxy-phenylisothiocyanat;3-carboxyphenyl isothiocyanate
CAS
2131-63-7
化学式
C8H5NO2S
mdl
MFCD00041367
分子量
179.199
InChiKey
PJRBPKOOGLKPFB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:165 °C
-
沸点:381.2±25.0 °C(Predicted)
-
密度:1.3522 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:81.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险类别码:R20/22,R36/37/38
-
危险品运输编号:2811
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
-
安全说明:S26,S36/37/39
-
储存条件:存储条件:2-8°C,密封保存,置于干燥处。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 间氨基苯甲酸 meta-aminobenzoic acid 99-05-8 C7H7NO2 137.138 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-甲氧基羰基异硫氰酸苯酯 methyl 3-isothiocyanatobenzoate 3125-66-4 C9H7NO2S 193.226 1-(3-羧苯基)-2-硫脲 3-thioureidobenzoic acid 37182-75-5 C8H8N2O2S 196.23
反应信息
-
作为反应物:描述:3-羧基异硫氰酸苯酯 在 溶剂黄146 、 间氯过氧苯甲酸 、 sodium hydroxide 作用下, 以 二氯甲烷 、 水 为溶剂, 反应 10.0h, 生成 3(3-carboxyphenyl)-2,4(1H,3H)-quinazolinedione参考文献:名称:4(3H)-喹唑啉酮抗菌剂结构空间的探索。摘要:我们在此报告了4(3H)-喹唑啉酮的79种衍生物的合成及其对耐甲氧西林的金黄色葡萄球菌(MRSA)的结构活性关系(SAR)。在体外测定中进一步评估了二十一种类似物。随后的药代动力学研究表明,化合物73((E)-3-(5-羧基-2-氟苯基)-2-(4-氰基苯乙烯基)喹唑啉-4(3H)-一)进一步研究。在临床相关的MRSA感染小鼠模型中,该化合物在体外和体内均与哌拉西林-他唑巴坦(TZP)协同作用。TZP组合缺乏抗MRSA的活性,但与化合物73协同作用以杀菌方式杀死MRSA。喹唑啉酮类药物与青霉素结合蛋白(PBP)2a的变构位点结合的能力使协同作用合理化,导致活性位点的开放,从而使β-内酰胺抗生素现在能够以其作用机理与活性位点结合。该组合可有效治疗MRSA感染,为此许多抗生素(包括TZP)已面临临床淘汰。DOI:10.1021/acs.jmedchem.0c00153
-
作为产物:描述:参考文献:名称:Rathke; Schaefer, Justus Liebigs Annalen der Chemie, 1873, vol. 169, p. 103摘要:DOI:
文献信息
-
Mechanism-Based Isocoumarin Inhibitors for Human Leukocyte Elastase. Effect of the 7-Amino Substituent and 3-Alkoxy Group in 3-Alkoxy-7-amino-4-chloroisocoumarins on Inhibitory Potency作者:John E. Kerrigan、Jozef Oleksyszyn、Chih-Min Kam、Joe Selzler、James C. PowersDOI:10.1021/jm00003a017日期:1995.2prepared and evaluated as inhibitors of human leukocyte elastase (HLE). In addition, a new series of acyl, urea, and carbamate derivatives of 7-amino-4-chloro-3-methoxyisocoumarin (1), 7-amino-4-chloro-3-propoxyisocoumarin (3), and 7-amino-4-chloro-3-(2-bromoethoxy)isocoumarin (6) have been synthesized. Most of the synthesized compounds are very potent inhibitors of HLE with kobs/[I] values between 10(4)已经制备了具有各种3-烷氧基取代基的一系列3-烷氧基-7-氨基-4-氯异香豆素,并将其评估为人白细胞弹性蛋白酶(HLE)的抑制剂。此外,还推出了一系列新的7-氨基-4-氯-3-甲氧基异香豆素(1),7-氨基-4-氯-3-丙氧基异香豆素(3)和7-氨基-香豆素的酰基,脲和氨基甲酸酯衍生物已经合成了4-氯-3-(2-溴乙氧基)异香豆素(6)。大多数合成的化合物都是非常有效的HLE抑制剂,其kobs / [I]值在10(4)和10(6)M-1 s-1之间。异香豆素环7-氨基位置的疏水取代基为HLE提供了最佳的选择性和抑制能力。在2-溴乙氧基系列中,具有PhNHCONH 7取代基的化合物24的kobs / [I]值为1.2 x 10(6)M-1 s-1,对HLE具有很高的选择性,并且是最有效的HLE抑制剂。经过HLE测试。在长链L-苯丙氨酰基衍生物中,kobs / [I]值为1.8 x 10(5)M-1
-
Substituted benzazoles and methods of their use as inhibitors of Raf kinase申请人:——公开号:US20040122237A1公开(公告)日:2004-06-24New substituted benz-azole compounds, compositions and methods of inhibition of Raf kinase activity in a human or animal subject are provided. The new compounds compositions may be used either alone or in combination with at least one additional agent for the treatment of a Raf kinase mediated disorder, such as cancer.提供了新的替代苯唑化合物、组合物和抑制人类或动物主体中Raf激酶活性的方法。这些新化合物组合物可以单独使用,也可以与至少一种额外药物结合,用于治疗由Raf激酶介导的疾病,如癌症。
-
2-Aminothiazole Derivatives as Selective Allosteric Modulators of the Protein Kinase CK2. 2. Structure-Based Optimization and Investigation of Effects Specific to the Allosteric Mode of Action作者:Benoît Bestgen、Irina Kufareva、Weiguang Seetoh、Chris Abell、Rolf W. Hartmann、Ruben Abagyan、Marc Le Borgne、Odile Filhol、Claude Cochet、Thierry Lomberget、Matthias EngelDOI:10.1021/acs.jmedchem.8b01765日期:2019.2.28Protein CK2 has gained much interest as an anticancer drug target in the past decade. We had previously described the identification of a new allosteric site on the catalytic α-subunit, along with first small molecule ligands based on the 4-(4-phenylthiazol-2-ylamino)benzoic acid scaffold. In the present work, structure optimizations guided by a binding model led to the identification of the lead compound在过去的十年中,蛋白CK2作为抗癌药物已引起了广泛的关注。先前我们已经描述了催化α-亚基上新的变构位点的识别,以及基于4-(4-苯基噻唑-2-基氨基)苯甲酸支架的第一个小分子配体的鉴定。在目前的工作中,以结合模型为指导的结构优化导致了铅化合物2-羟基-4-((4-(萘-2-基)噻唑-2-基)氨基)苯甲酸的鉴定(27) ,显示了对纯化的CK2α的亚微摩尔效价(IC50 = 0.6μM)。此外,与ATP竞争性候选药物CX-4945相比,27种诱导786-O肾细胞癌细胞的凋亡和细胞死亡(EC50 = 5μM)甚至更有效地抑制STAT3激活(EC50为1.6μM对5.3μM)。尤其,我们的变构配体抑制CK2的能力因各个底物而异。总之,新的变构口袋被证明是可药物治疗的部位,为开发有效和选择性的变构CK2抑制剂提供了绝佳的前景。
-
Correlation of carbon-13 substituent-induced chemical shifts:Meta- andpara-substituted methyl benzoates作者:Miloš Buděšńský、Otto ExnerDOI:10.1002/mrc.1260270612日期:1989.6Carbon‐13 NMR spectra are reported for 69 substituted methyl benzoates in deuteriochloroform or in its mixture with dimethyl sulphoxide‐d6. The substituent‐induced chemical shifts (SCS) of the CO carbon correlate poorly with dual substituent parameters (DSP) in all possible modifications, and for meta derivatives in particular this correlation is both overpara meterized and imprecise. A much better
-
METHOD FOR PRODUCING ISOTHIOCYANATE COMPOUND申请人:Takada Junko公开号:US20130245305A1公开(公告)日:2013-09-19The object of the present invention is to provide a novel method for producing an isothiocyanate compound having a carboxyl group(s) by a reaction of the corresponding amino compound having a carboxyl group(s), thiocarbonyldiimidazole and a base, in one step with high purity. An amino compound having a carboxyl group(s) is reacted with thiocarbonyldiimidazole in a solvent in the presence of a base to obtain an isothiocyanate compound having a carboxyl group(s).
表征谱图
-
氢谱1HNMR
-
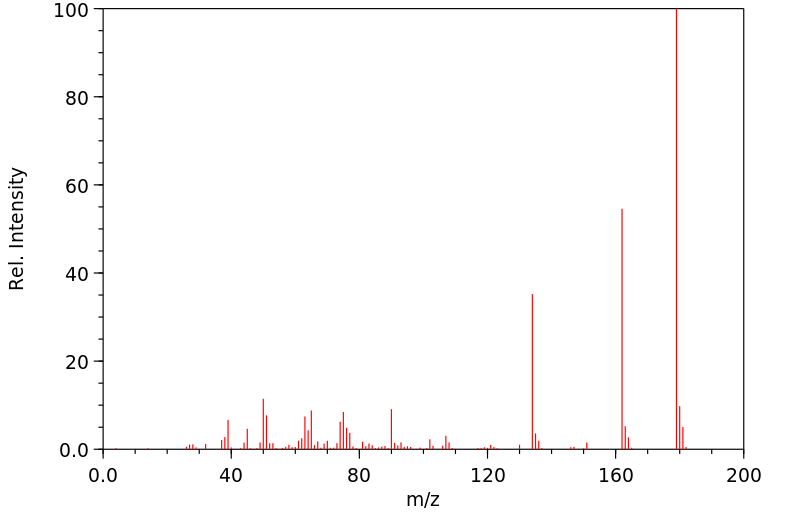
质谱MS
-
碳谱13CNMR
-
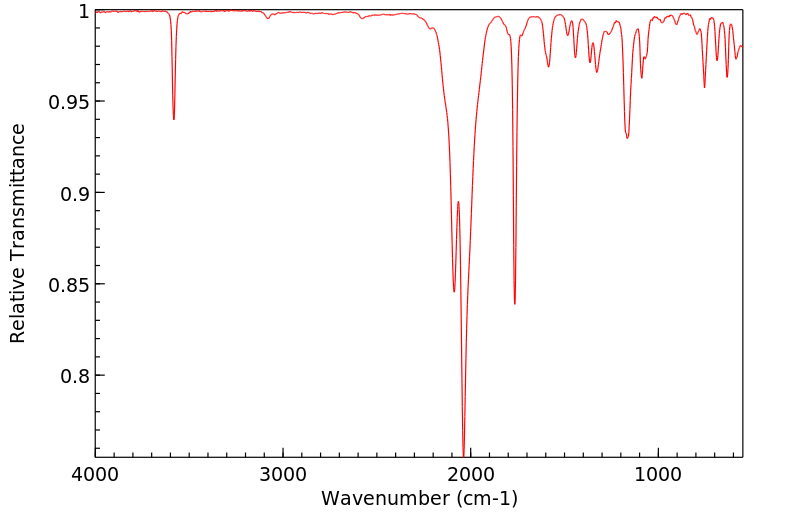
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫